Nucleosomes accelerate transcription factor dissociation
- PMID: 24353316
- PMCID: PMC3950707
- DOI: 10.1093/nar/gkt1319
Nucleosomes accelerate transcription factor dissociation
Abstract
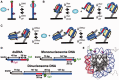
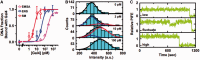
Transcription factors (TF) bind DNA-target sites within promoters to activate gene expression. TFs target their DNA-recognition sequences with high specificity by binding with resident times of up to hours in vitro. However, in vivo TFs can exchange on the order of seconds. The factors that regulate TF dynamics in vivo and increase dissociation rates by orders of magnitude are not known. We investigated TF binding and dissociation dynamics at their recognition sequence within duplex DNA, single nucleosomes and short nucleosome arrays with single molecule total internal reflection fluorescence (smTIRF) microscopy. We find that the rate of TF dissociation from its site within either nucleosomes or nucleosome arrays is increased by 1000-fold relative to duplex DNA. Our results suggest that TF binding within chromatin could be responsible for the dramatic increase in TF exchange in vivo. Furthermore, these studies demonstrate that nucleosomes regulate DNA-protein interactions not only by preventing DNA-protein binding but by dramatically increasing the dissociation rate of protein complexes from their DNA-binding sites.
Figures




References
-
- Serikawa Y, Shirakawa M, Matsuo H, Kyogoku Y. Efficient expression and Zn(II)-dependent structure of the DNA binding domain of the yeast GAL4 protein. Protein Eng. 1990;3:267–272. - PubMed
-
- Rasnik I, McKinney SA, Ha T. Nonblinking and long-lasting single-molecule fluorescence imaging. Nat. Methods. 2006;3:891–893. - PubMed
-
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. - PubMed
-
- Richmond TJ, Davey CA. The structure of DNA in the nucleosome core. Nature. 2003;423:145–150. - PubMed
-
- Lin YS, Carey MF, Ptashne M, Green MR. GAL4 derivatives function alone and synergistically with mammalian activators in vitro. Cell. 1988;54:659–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

