Indacaterol therapy in moderate-to-severe chronic obstructive pulmonary disease: findings from a single-center primary care cohort
- PMID: 24353411
- PMCID: PMC3862397
- DOI: 10.2147/COPD.S53707
Indacaterol therapy in moderate-to-severe chronic obstructive pulmonary disease: findings from a single-center primary care cohort
Abstract
Background: Once-daily long-acting β2-agonists (LABAs) are an important treatment option, either alone or in combination with other inhaled long-acting bronchodilators in the management of chronic obstructive pulmonary disease (COPD).
Aims/objectives: To audit the effectiveness of indacaterol as maintenance therapy in patients with moderate-to-severe COPD (Global initiative for chronic Obstructive Lung Disease [GOLD] stage II/III).
Methods: This was a single-center audit of a primary care COPD cohort comprising all patients treated with indacaterol following treatment escalation (as per National Institute for Health and Care Excellence guidelines) or failure with other therapies. The sample was restricted to patients treated for a minimum of 12 months with indacaterol, for whom preswitching and follow-up spirometry as well as exacerbation frequency data were available (GOLD spirometry guidelines). Pulmonary function was assessed by spirometry (recorded as forced expiratory volume in 1 second [FEV1] expressed as percentage predicted). Relevant self-reported qualitative information was recorded in descriptive terms for quality of life (QoL) assessment.
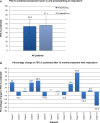
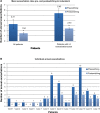
Results: A total of 15 patients met the audit inclusion criteria (66.6% male, mean age 64.9±7.7 years). COPD disease duration ranged from 1 to 22 years; 93% had GOLD stage II or III COPD. Follow-up ranged in duration from 12 to 27 months. Indacaterol was associated with a significant reduction in exacerbation frequency compared with the 12 months prior to initiation (P=0.02). In those patients who experienced three or more exacerbations/year, mean exacerbation rate fell from 5.43±1.07 to 2.43±0.2 after 12 months treatment with indacaterol (P=0.02). A reduction in dyspnea was noted in 53% of patients. Similarly, improvements in exercise tolerance and well-being were self-reported in 67% and 93%, respectively.
Conclusion: Indacaterol was found to be an effective LABA as an escalation or switch medication in patients with moderate-to-severe COPD. Indacaterol was effective both as monotherapy and in combination with a long-acting muscarinic antagonist. Switching to indacaterol from a LABA/inhaled corticosteroid fixed-combination inhaler significantly reduced the number of acute exacerbations and also improved self-reported QoL.
Keywords: audit; bronchodilators; chronic obstructive pulmonary disease; effectiveness; indacaterol; primary care.
Figures
Similar articles
-
LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD.Int J Chron Obstruct Pulmon Dis. 2015 Jun 5;10:1015-26. doi: 10.2147/COPD.S84436. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26082625 Free PMC article. Clinical Trial.
-
Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial.Respir Res. 2017 Jul 18;18(1):140. doi: 10.1186/s12931-017-0622-x. Respir Res. 2017. PMID: 28720132 Free PMC article. Clinical Trial.
-
Efficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study.Int J Chron Obstruct Pulmon Dis. 2013;8:501-8. doi: 10.2147/COPD.S49615. Epub 2013 Oct 17. Int J Chron Obstruct Pulmon Dis. 2013. PMID: 24159259 Free PMC article. Clinical Trial.
-
Comparative efficacy of indacaterol in chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2012;7:145-52. doi: 10.2147/COPD.S19805. Epub 2012 Mar 5. Int J Chron Obstruct Pulmon Dis. 2012. PMID: 22419862 Free PMC article. Review.
-
Clinical role of dual bronchodilation with an indacaterol-glycopyrronium combination in the management of COPD: its impact on patient-related outcomes and quality of life.Int J Chron Obstruct Pulmon Dis. 2015 Jul 23;10:1383-92. doi: 10.2147/COPD.S55488. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26229457 Free PMC article. Review.
Cited by
-
A real-world evaluation of indacaterol and other bronchodilators in COPD: the INFLOW study.Int J Chron Obstruct Pulmon Dis. 2015 Oct 5;10:2109-20. doi: 10.2147/COPD.S83071. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26491281 Free PMC article.
References
-
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. - PubMed
-
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. - PubMed
-
- O’Reilly J, Jones MM, Parnham J, Lovibond K, Rudolf M. Management of stable chronic obstructive pulmonary disease in primary and secondary care: summary of updated NICE guidance. BMJ. 2010;340:c3134. - PubMed
-
- National Institute for Health and Care Excellence Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. 2010. [Accessed October 18, 2013]. Available from: http://www.nice.org.uk/nicemedia/live/13029/49425/49425.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

