The Enantioselective Addition of Alkyne Nucleophiles to Carbonyl Groups
- PMID: 24353484
- PMCID: PMC3864370
- DOI: 10.1002/adsc.200800776
The Enantioselective Addition of Alkyne Nucleophiles to Carbonyl Groups
Abstract
Over the past decade, large strides have been achieved in the invention of methods for the direct enantioselective addition of alkynes and metal alkynylide nucleophiles into prochiral aldehydes, ketones, and imines. This review highlights and compares the available methods for these transformations.
Keywords: alkynylation; catalytic asymmetric; propargylic alcohol; propargylic amine.
Figures
















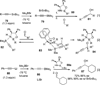
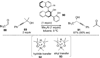
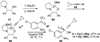
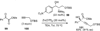

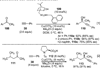
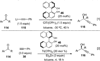

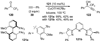

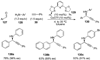
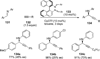



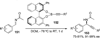

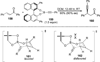


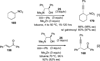


References
-
- Stang PJ, Diederich F, editors. Modern Acetylene Chemistry. Weinheim: VCH; 1995.
-
-
For a [2+2+2]: Witulski B, Zimmermann A, Gowans ND. Chem. Commun. 2002:2984–2985.
-
-
-
For a review on hydrometallations, see: Trost BM, Ball ZT. Synthesis. 2005:853–887.
-
-
-
For some representative examples, see: Roethle PA, Trauner D. Org. Lett. 2006;8:345. Trost BM, Mueller TJJ, Martinez J. J. Am. Chem. Soc. 1995;117:1888–1899. Zhu G, Lu X. J. Org. Chem. 1995;60:1087. See also, references and .
-
-
- Fleming JJ, Du Bois J. J. Am. Chem. Soc. 2006;128:3926. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
