A microchip electrophoresis-mass spectrometric platform for fast separation and identification of enantiomers employing the partial filling technique
- PMID: 24354006
- PMCID: PMC4097118
- DOI: 10.1016/j.chroma.2013.10.020
A microchip electrophoresis-mass spectrometric platform for fast separation and identification of enantiomers employing the partial filling technique
Abstract
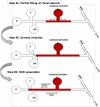
A microchip electrophoresis-mass spectrometric (MCE-MS) method was developed for fast chiral analysis. The proposed MCE-MS platform deployed a glass/PDMS hybrid microchip with an easy-to-fabricate monolithic nanoelectrospray emitter. Enantiomeric MCE separation was achieved by means of the partial filling technique. A novel chip design with an arm channel connecting to the middle of the MCE separation channel for delivering the chiral selector was tested and proven valid. Enantiomeric separation of3.4-dihydroxyphenylalanine (DOPA), glutamic acid (Glu), and serine (Ser), the selected test compounds,were achieved within 130 s with resolution values (R(s)) of 2.4, 1.1, and 1.0, respectively. The proposed chiral MCE-MS assay was sensitive and had detection limits of 43 nM for l-DOPA and 47 nM for d-DOPA.The analytical platform was well suited for studies of stereochemical preference in living cells because it integrated cell culture, sample injection, chiral separation, and MS detection into a single platform.Metabolism of DOPA in human SH-SY5Y neuronal cells was studied as a model system. On-chip incubation of SH-SY5Y cells with racemic DOPA was carried out, and the incubation solution was injected and in-line assayed at time intervals. It was found that l-DOPA concentration decreased gradually as incubation time increased while the concentration of coexisting d-DOPA remained constant. The results firmly indicated that SH-SY5Y cells metabolized l-DOPA effectively while left d-DOPA intact.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

