Can too many copies spoil the broth?
- PMID: 24354594
- PMCID: PMC3878197
- DOI: 10.1186/1475-2859-12-128
Can too many copies spoil the broth?
Abstract
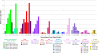
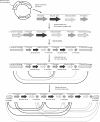
The success of Pichia pastoris as a heterologous expression system lies predominantly in the impressive yields that can be achieved due to high volumetric productivity. However, low specific productivity still inhibits the potential success of this platform. Multi-(gene) copy clones are potentially a quick and convenient method to increase recombinant protein titer, yet they are not without their pitfalls. It has been more than twenty years since the first reported use of multi-copy clones and it is still an active area of research to find the fastest and most efficient method for generating these strains. It has also become apparent that there is not always a linear correlation between copy number and protein titer, leading to in-depth investigations into how to minimize the negative impact of secretory stress and achieve clonal stability.
Figures

References
-
- Cregg JM. Pichia Protocols. 2. Totowa, New Jersey: Humana Press; 2007.
-
- Dale C. Pichia pastoris: a eukaryotic system for the large-scale production of biopharmaceuticals. Biopharm. 1999;12:36.
-
- Romanos M, Scorer C, Sreekrishna K, Clare J. In: Pichia Protocols, Volume 103. Higgins DR, Cregg J, editor. Humana Press; 1998. The Generation of Multicopy Recombinant Strains; pp. 55–72. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

