The 4-cysteine zinc-finger motif of the RNA polymerase regulator DksA serves as a thiol switch for sensing oxidative and nitrosative stress
- PMID: 24354846
- PMCID: PMC4053250
- DOI: 10.1111/mmi.12498
The 4-cysteine zinc-finger motif of the RNA polymerase regulator DksA serves as a thiol switch for sensing oxidative and nitrosative stress
Abstract
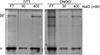
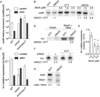
We show that thiols in the 4-cysteine zinc-finger motif of DksA, an RNA polymerase accessory protein known to regulate the stringent response, sense oxidative and nitrosative stress. Hydrogen peroxide- or nitric oxide (NO)-mediated modifications of thiols in the DksA 4-cysteine zinc-finger motif release the metal cofactor and drive reversible changes in the α-helicity of the protein. Wild-type and relA spoT mutant Salmonella, but not isogenic dksA-deficient bacteria, experience the downregulation of r-protein and amino acid transport expression after NO treatment, suggesting that DksA can regulate gene expression in response to NO congeners independently of the ppGpp alarmone. Oxidative stress enhances the DksA-dependent repression of rpsM, while preventing the activation of livJ and hisG gene transcription that is supported by reduced, zinc-bound DksA. The inhibitory effects of oxidized DksA on transcription are reversible with dithiothreitol. Our investigations indicate that sensing of reactive species by DksA redox active thiols fine-tunes the expression of translational machinery and amino acid assimilation and biosynthesis in accord with the metabolic stress imposed by oxidative and nitrosative stress. Given the conservation of Cys(114) , and neighbouring hydrophobic and charged amino acids in DksA orthologues, phylogenetically diverse microorganisms may use the DksA thiol switch to regulate transcriptional responses to oxidative and nitrosative stress.
© 2013 John Wiley & Sons Ltd.
Conflict of interest statement
The authors do not have a conflict of interest to declare.
Figures


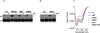

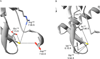
References
-
- Aberg A, Shingler V, Balsalobre C. Regulation of the fimB promoter: a case of differential regulation by ppGpp and DksA in vivo. Mol Microbiol. 2008;67:1223–1241. - PubMed
-
- Bae JB, Park JH, Hahn MY, Kim MS, Roe JH. Redox-dependent changes in RsrA, an anti-sigma factor in Streptomyces coelicolor: zinc release and disulfide bond formation. J Mol Biol. 2004;335:425–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I01 BX002073/BX/BLRD VA/United States
- R01 AI073971/AI/NIAID NIH HHS/United States
- AI052066/AI/NIAID NIH HHS/United States
- R01 AI039557/AI/NIAID NIH HHS/United States
- AI039557/AI/NIAID NIH HHS/United States
- T32 GM008730/GM/NIGMS NIH HHS/United States
- R56 AI054959/AI/NIAID NIH HHS/United States
- R01 AI044486/AI/NIAID NIH HHS/United States
- R01 AI054959/AI/NIAID NIH HHS/United States
- AI077645/AI/NIAID NIH HHS/United States
- AI073971/AI/NIAID NIH HHS/United States
- T32 AI052066/AI/NIAID NIH HHS/United States
- R01 AI083646/AI/NIAID NIH HHS/United States
- AI083646/AI/NIAID NIH HHS/United States
- R56 AI077645/AI/NIAID NIH HHS/United States
- R01 AI052237/AI/NIAID NIH HHS/United States
- R01 AI075093/AI/NIAID NIH HHS/United States
- AI54959/AI/NIAID NIH HHS/United States
- AI052237/AI/NIAID NIH HHS/United States
- AI075093/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

