Preferential secretion of inducible HSP70 by vitiligo melanocytes under stress
- PMID: 24354861
- PMCID: PMC3947476
- DOI: 10.1111/pcmr.12208
Preferential secretion of inducible HSP70 by vitiligo melanocytes under stress
Abstract
Inducible HSP70 (HSP70i) chaperones peptides from stressed cells, protecting them from apoptosis. Upon extracellular release, HSP70i serves an adjuvant function, enhancing immune responses to bound peptides. We questioned whether HSP70i differentially protects control and vitiligo melanocytes from stress and subsequent immune responses. We compared expression of HSP70i in skin samples, evaluated the viability of primary vitiligo and control melanocytes exposed to bleaching phenols, and measured secreted HSP70i. We determined whether HSP70i traffics to melanosomes to contact immunogenic proteins by cell fractionation, western blotting, electron microscopy, and confocal microscopy. Viability of vitiligo and control melanocytes was equally affected under stress. However, vitiligo melanocytes secreted increased amounts of HSP70i in response to MBEH, corroborating with aberrant HSP70i expression in patient skin. Intracellular HSP70i colocalized with melanosomes, and more so in response to MBEH in vitiligo melanocytes. Thus, whereas either agent is cytotoxic to melanocytes, MBEH preferentially induces immune responses to melanocytes.
Keywords: autoimmune; heat shock protein; melanocyte; stress; vitiligo.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

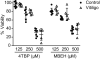




Similar articles
-
HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo.J Invest Dermatol. 2008 Aug;128(8):2041-8. doi: 10.1038/jid.2008.45. Epub 2008 Mar 13. J Invest Dermatol. 2008. PMID: 18337834 Free PMC article.
-
Enhanced bleaching treatment: opportunities for immune-assisted melanocyte suicide in vitiligo.Exp Dermatol. 2014 Aug;23(8):529-33. doi: 10.1111/exd.12449. Epub 2014 Jul 10. Exp Dermatol. 2014. PMID: 24840876 Free PMC article.
-
Monobenzyl ether of hydroquinone and 4-tertiary butyl phenol activate markedly different physiological responses in melanocytes: relevance to skin depigmentation.J Invest Dermatol. 2010 Jan;130(1):211-20. doi: 10.1038/jid.2009.214. J Invest Dermatol. 2010. PMID: 19657355
-
A central role for inducible heat-shock protein 70 in autoimmune vitiligo.Exp Dermatol. 2013 Sep;22(9):566-9. doi: 10.1111/exd.12183. Epub 2013 Jun 20. Exp Dermatol. 2013. PMID: 23786523 Free PMC article. Review.
-
The impaired unfolded protein-premelanosome protein and transient receptor potential channels-autophagy axes in apoptotic melanocytes in vitiligo.Pigment Cell Melanoma Res. 2022 Jan;35(1):6-17. doi: 10.1111/pcmr.13006. Epub 2021 Aug 10. Pigment Cell Melanoma Res. 2022. PMID: 34333860 Review.
Cited by
-
The convergence theory for vitiligo: A reappraisal.Exp Dermatol. 2019 Jun;28(6):647-655. doi: 10.1111/exd.13677. Epub 2018 Jun 28. Exp Dermatol. 2019. PMID: 29704874 Free PMC article. Review.
-
Optimization of Monobenzone-Induced Vitiligo Mouse Model by the Addition of Chronic Stress.Int J Mol Sci. 2023 Apr 10;24(8):6990. doi: 10.3390/ijms24086990. Int J Mol Sci. 2023. PMID: 37108153 Free PMC article.
-
Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation.Antioxidants (Basel). 2025 May 7;14(5):555. doi: 10.3390/antiox14050555. Antioxidants (Basel). 2025. PMID: 40427437 Free PMC article. Review.
-
The relationship between stress and vitiligo: Evaluating perceived stress and electronic medical record data.PLoS One. 2020 Jan 27;15(1):e0227909. doi: 10.1371/journal.pone.0227909. eCollection 2020. PLoS One. 2020. PMID: 31986193 Free PMC article.
-
Damage-associated molecular patterns in vitiligo: igniter fuse from oxidative stress to melanocyte loss.Redox Rep. 2022 Dec;27(1):193-199. doi: 10.1080/13510002.2022.2123864. Redox Rep. 2022. PMID: 36154894 Free PMC article. Review.
References
-
- Abdou AG, Maraee AH, Reyad W. Immunohistochemical expression of heat shock protein 70 in vitiligo. Ann Diagn Pathol. 2013;17:245–249. - PubMed
-
- Agarraberes FA, Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114:2491–2499. - PubMed
-
- Ando H, Niki Y, Ito M, Akiyama K, Matsui MS, Yarosh DB, Ichihashi M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol. 2012;132:1222–1229. - PubMed
-
- Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. - PubMed
-
- Beere HM, Green DR. Stress management - heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

