Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis
- PMID: 24354889
- PMCID: PMC4168386
- DOI: 10.1111/bcp.12313
Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis
Abstract
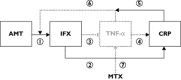
Aims: Infliximab, an anti-tumour necrosis factor-α monoclonal antibody, is indicated in rheumatoid arthritis (RA). Our objective was to evaluate the influence of the sources of infliximab pharmacokinetic variability in RA.
Methods: Eighty-four patients treated with infliximab for RA were included in a prospective noncomparative study. They were analysed between two consecutive infliximab infusions. Infliximab concentrations were measured before the infusion, 2 h, 1 and 4 weeks after the infusion and immediately before the next infusion. Infliximab concentrations were described using a two-compartment population pharmacokinetic model.
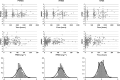
Results: The mean (interindividual standard deviation) estimated central volume of distribution was 2.3 l (36%) and systemic clearance was 0.019 l h(-1) (37%). The central volume of distribution increased with bodyweight; it was doubled between 50 and 90 kg. Systemic clearance increased with pre-infusion C-reactive protein concentration by 20%, varying from 3 to 14 mg l(-) 1, and was decreased by 30% when methotrexate was coadministered.
Conclusions: The influence of methotrexate and inflammation on infliximab clearance suggests that individual adjustment of infliximab doses according to disease activity may be useful in RA.
Keywords: inflammation; infliximab; monoclonal antibodies; pharmacokinetics; rheumatoid arthritis.
© 2013 The British Pharmacological Society.
Figures



Comment in
-
A robust estimation of infliximab pharmacokinetic parameters in Crohn's disease.Eur J Clin Pharmacol. 2015 Dec;71(12):1541-2. doi: 10.1007/s00228-015-1942-8. Epub 2015 Sep 15. Eur J Clin Pharmacol. 2015. PMID: 26369535 No abstract available.
Similar articles
-
Chimeric anti-tumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy.J Rheumatol. 2000 Apr;27(4):841-50. J Rheumatol. 2000. PMID: 10782805 Clinical Trial.
-
Golimumab pharmacokinetics after repeated subcutaneous and intravenous administrations in patients with rheumatoid arthritis and the effect of concomitant methotrexate: an open-label, randomized study.Clin Ther. 2012 Jan;34(1):77-90. doi: 10.1016/j.clinthera.2011.11.015. Epub 2011 Dec 14. Clin Ther. 2012. PMID: 22169051 Clinical Trial.
-
A relationship between pharmacokinetics (PK) and the efficacy of infliximab for patients with rheumatoid arthritis: characterization of infliximab-resistant cases and PK-based modified therapy.Mod Rheumatol. 2007;17(2):83-91. doi: 10.1007/s10165-006-0544-9. Epub 2007 Apr 20. Mod Rheumatol. 2007. PMID: 17437161 Clinical Trial.
-
Clinical pharmacokinetics and use of infliximab.Clin Pharmacokinet. 2007;46(8):645-60. doi: 10.2165/00003088-200746080-00002. Clin Pharmacokinet. 2007. PMID: 17655372 Review.
-
Infliximab treatment of rheumatoid arthritis and Crohn's disease.Ann Pharmacother. 2003 Sep;37(9):1256-65. doi: 10.1345/aph.1C039. Ann Pharmacother. 2003. PMID: 12921510 Review.
Cited by
-
Practical recommendations for the use of therapeutic drug monitoring of biopharmaceuticals in inflammatory diseases.Clin Pharmacol. 2017 Oct 3;9:101-111. doi: 10.2147/CPAA.S138414. eCollection 2017. Clin Pharmacol. 2017. PMID: 29042821 Free PMC article. Review.
-
Calprotectin strongly and independently predicts relapse in rheumatoid arthritis and polyarticular psoriatic arthritis patients treated with tumor necrosis factor inhibitors: a 1-year prospective cohort study.Arthritis Res Ther. 2018 Dec 13;20(1):275. doi: 10.1186/s13075-018-1764-z. Arthritis Res Ther. 2018. PMID: 30545393 Free PMC article.
-
Characterizing Pharmacokinetics in Children With Obesity-Physiological, Drug, Patient, and Methodological Considerations.Front Pharmacol. 2022 Mar 10;13:818726. doi: 10.3389/fphar.2022.818726. eCollection 2022. Front Pharmacol. 2022. PMID: 35359853 Free PMC article. Review.
-
Methotrexate Reduces the Probability of Discontinuation of TNF Inhibitors in Seropositive Patients With Rheumatoid Arthritis. A Real-World Data Analysis.Front Med (Lausanne). 2021 Jun 29;8:692557. doi: 10.3389/fmed.2021.692557. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34268325 Free PMC article.
-
A pharmacokinetic comparison of anrukinzumab, an anti- IL-13 monoclonal antibody, among healthy volunteers, asthma and ulcerative colitis patients.Br J Clin Pharmacol. 2015 Jul;80(1):101-9. doi: 10.1111/bcp.12589. Epub 2015 Jun 1. Br J Clin Pharmacol. 2015. PMID: 25614144 Free PMC article. Clinical Trial.
References
-
- St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, Keystone EC. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451–1459. - PubMed
-
- Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006;54:3782–3789. - PubMed
-
- Mulleman D, Chu Miow Lin D, Ducourau E, Emond P, Ternant D, Magdelaine-Beuzelin C, Valat JP, Paintaud G, Goupille P. Trough infliximab concentrations predict efficacy and sustained control of disease activity in rheumatoid arthritis. Ther Drug Monit. 2010;32:232–236. - PubMed
-
- Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, Dijkmans BA, Aarden L. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:704–707. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

