A multicentre randomized controlled trial of an empowerment-inspired intervention for adolescents starting continuous subcutaneous insulin infusion--a study protocol
- PMID: 24354899
- PMCID: PMC3879650
- DOI: 10.1186/1471-2431-13-212
A multicentre randomized controlled trial of an empowerment-inspired intervention for adolescents starting continuous subcutaneous insulin infusion--a study protocol
Abstract
Background: Continuous subcutaneous insulin infusion (CSII) treatment among children with type 1 diabetes is increasing in Sweden. However, studies evaluating glycaemic control in children using CSII show inconsistent results. The distribution of responsibility for diabetes self-management between children and parents is often unclear and needs clarification. There is much published support for continued parental involvement and shared diabetes management during adolescence. Guided Self-Determination (GSD) is an empowerment-based, person-centred, reflection and problem solving method intended to guide the patient to become self-sufficient and develop life skills for managing difficulties in diabetes self-management. This method has been adapted for adolescents and parents as Guided Self-Determination-Young (GSD-Y). This study aims to evaluate the effect of an intervention with GSD-Y in groups of adolescents starting on insulin pumps and their parents on diabetes-related family conflicts, perceived health and quality of life (QoL), and metabolic control. Here, we describe the protocol and plans for study enrollment.
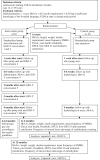
Methods/design: This study is designed as a randomized, controlled, prospective, multicentre study. Eighty patients between 12-18 years of age who are planning to start CSII will be included. All adolescents and their parents will receive standard insulin pump training. The education intervention will be conducted when CSII is to be started and at four appointments in the first 4 months after starting CSII. The primary outcome is haemoglobin A1c levels. Secondary outcomes are perceived health and QoL, frequency of blood glucose self-monitoring and bolus doses, and usage of carbohydrate counting. The following instruments will be used: Disabkids, 'Check your health', the Diabetes Family Conflict Scale and the Swedish Diabetes Empowerment Scale. Outcomes will be evaluated within and between groups by comparing data at baseline, and at 6 and 12 months after starting treatment.
Discussion: In this study, we will assess the effect of starting an CSII together with the model of GSD to determine whether this approach leads to retention of improved glycaemic control, QoL, responsibility distribution and reduced diabetes-related conflicts in the family.
References
-
- SWEDIABKIDS. Nationellt register för barn- och ungdomsdiabetes, Årsrapport, 2011 års resultat. 2011. https://www.ndr.nu/NDR2/ShowPDF.aspx?Document=NDR-Child/AnnualReport-201....
-
- International Diabetes Federation. Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence. 2011. http://www.ispad.org/sites/default/files/resources/files/idf-ispad_diabe....
-
- Sjöblad S. Barn- och ungdomsdiabetes. Lund: Studentlitteratur; 2008. 2. [uppdaterade och utvidgade] uppl. edn.
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;13(14):977–986. - PubMed
-
- Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1994;13(2):177–188. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical