Modulation of synaptic transmission by adenosine in layer 2/3 of the rat visual cortex in vitro
- PMID: 24355495
- PMCID: PMC3960601
- DOI: 10.1016/j.neuroscience.2013.12.018
Modulation of synaptic transmission by adenosine in layer 2/3 of the rat visual cortex in vitro
Abstract
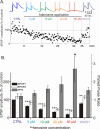
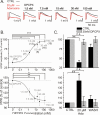
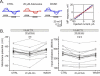
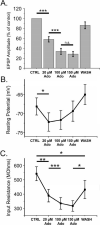
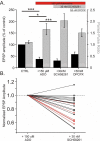
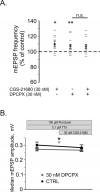
Adenosine is a wide-spread endogenous neuromodulator. In the central nervous system it activates A1 and A2A receptors (A1Rs and A2ARs) which have differential distributions, different affinities to adenosine, are coupled to different G-proteins, and have opposite effects on synaptic transmission. Although effects of adenosine are studied in detail in several brain areas, such as the hippocampus and striatum, the heterogeneity of the effects of A1R and A2AR activation and their differential distribution preclude generalization over brain areas and cell types. Here we study adenosine's effects on excitatory synaptic transmission to layer 2/3 pyramidal neurons in slices of the rat visual cortex. We measured effects of bath application of adenosine receptor ligands on evoked excitatory postsynaptic potentials (EPSPs), miniature excitatory postsynaptic potentials (mEPSPs), and membrane properties. Adenosine reduced the amplitude of evoked EPSPs and excitatory postsynaptic currents (EPSCs), and reduced frequency of mEPSPs in a concentration-dependent and reversible manner. Concurrent with EPSP/C amplitude reduction was an increase in the paired-pulse ratio. These effects were blocked by application of the selective A1R antagonist DPCPX (8-cyclopentyl-1,3-dipropylxanthine), suggesting that activation of presynaptic A1Rs suppresses excitatory transmission by reducing release probability. Adenosine (20μM) hyperpolarized the cell membrane from -65.3±1.5 to -67.7±1.8mV, and reduced input resistance from 396.5±44.4 to 314.0±36.3MOhm (∼20%). These effects were also abolished by DPCPX, suggesting postsynaptic A1Rs. Application of the selective A2AR antagonist SCH-58261 (2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-a-mine) on the background of high adenosine concentrations revealed an additional decrease in EPSP amplitude. Moreover, application of the A2AR agonist CGS-21680 (4-[2-[[6-amino-9-(N-ethyl-β-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride) led to an A1R-dependent increase in mEPSP frequency. Dependence of the A2AR effects on the A1R availability suggests interaction between these receptors, whereby A2ARs exert their facilitatory effect on synaptic transmission by inhibiting the A1R-mediated suppression. Our results demonstrate functional pre and postsynaptic A1Rs and presynaptic A2ARs in layer 2/3 of the visual cortex, and suggest interaction between presynaptic A2ARs and A1Rs.
Keywords: 2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-a-mine (A(2A)R antagonist); 4-[2-[[6-amino-9-(N-ethyl-β-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride (A(2A)R agonist); 8-cyclopentyl-1,3-dipropylxanthine (A(1)R antagonist); A(1) and A(2A) receptors; A(1)R; A(2A)R; APV; ATP; Ado; CGS-21680; DPCPX; EPSC; EPSP; SCH-58261; TTX; adenosine; adenosine receptor type 1; adenosine receptor type 2A; adenosine triphosphate; d-(−)-2-amino-5-phosphonopentanoic acid; excitatory postsynaptic current; excitatory postsynaptic potential; mEPSP; miniature excitatory postsynaptic potential; modulation; neocortex; presynaptic; synaptic transmission; tetrodotoxin.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




References
-
- Brand A, Vissiennon Z, Eschke D, Nieber K. Adenosine A1 and A3 receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85–95. - PubMed
-
- Chaudhuri A, Cohen RZ, Larocque S. Distribution of adenosine A1 receptors in primary visual cortex of developing and adult monkeys. Exp. Brain Res. 1998;123:351–354. - PubMed
-
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26(7):2080–2087. - PMC - PubMed
-
- Ciruela F, Gómez-Soler M, Guidolin D, Borroto-Escuela D, Agnati L, Fuxe K, Fernández-Dueñas V. Adenosine receptor containing oligomers: Their role in the control of dopamine and glutamate neurotransmission in the brain. Biochim. Biophys. Acta. 2011;1808:1245–1255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

