Primary hip replacement prostheses and their evidence base: systematic review of literature
- PMID: 24355538
- PMCID: PMC3898711
- DOI: 10.1136/bmj.f6956
Primary hip replacement prostheses and their evidence base: systematic review of literature
Abstract
Objective: To determine the extent to which prostheses with no readily available evidence to support their use are being implanted in primary total hip arthroplasty.
Design: Systematic review of the literature.
Data sources: The 9th annual report of the National Joint Registry of England and Wales (NJR) was analysed to identify prostheses with an Orthopaedic Data Evaluation Panel rating of "unclassified" or "pre-entry" used in primary total hip arthroplasty in 2011. A systematic review of those prostheses was carried out using PubMed, Cochrane, Embase, OVID, and Google databases.
Study selection: Prostheses used in primary total hip arthroplasty as published in the NJR's 9th annual report were analysed. Only literature that included the name of the prosthesis was included. Literature yielded in the search results was excluded if it reported animal, non-orthopaedic, non-total hip arthroplasty, or non-device related studies.
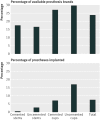
Results: The systematic review found that 24% (57/235) of all hip replacement implants available to surgeons in the UK have no evidence for their clinical effectiveness. It also shows that 10,617 (7.8%) of the 136,593 components used in primary hip replacements in 2011 were implanted without readily identifiable evidence of clinical effectiveness. These comprised 157 cemented stems (0.5% of 34,655 implanted), 936 (2.8% of 33,367) uncemented stems, 1732 (7.1% of 24,349) cemented cups, and 7577 (17.1% of 44,222) uncemented cups.
Conclusions: This study shows that a considerable proportion of prostheses available to orthopaedic surgeons have no readily available evidence of clinical effectiveness to support their use. Concern exists about the current system of device regulation, and the need for a revised process for introducing new orthopaedic devices is highlighted.
Conflict of interest statement
Competing interests:. All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
New and unproved medical devices.BMJ. 2013 Dec 19;347:f7413. doi: 10.1136/bmj.f7413. BMJ. 2013. PMID: 24355541 No abstract available.
References
-
- Wilmshurst P. The regulation of medical devices. BMJ 2011;342:d2822. - PubMed
-
- European Commission. Exploring innovative healthcare—the role of medical technology innovation and regulation. European Commission, 2011.
-
- Sorrel S. Medical device development: U.S. and EU differences. 2006. http://appliedclinicaltrialsonline.findpharma.com/appliedclinicaltrials/....
-
- Cohen D. Out of joint: the story of the ASR. BMJ 2011;342:d2905. - PubMed
-
- McCulloch P. The EU’s system for regulating medical devices. BMJ 2012;345:e7126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
