Nuclear mechanics and mechanotransduction in health and disease
- PMID: 24355792
- PMCID: PMC3883624
- DOI: 10.1016/j.cub.2013.11.009
Nuclear mechanics and mechanotransduction in health and disease
Abstract
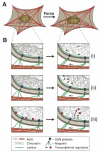
The nucleus is the defining feature of eukaryotic cells and often represents the largest organelle. Over the past decade, it has become apparent that the nucleus is tightly integrated into the structural network of the cell through so-called LINC (linker of the nucleoskeleton and cytoskeleton) complexes, which enable transmission of forces between the nucleus and cytoskeleton. This physical connection between the nucleus and the cytoskeleton is essential for a broad range of cellular functions, including intracellular nuclear movement and positioning, cytoskeletal organization, cell polarization, and cell migration. Recent reports further indicate that forces transmitted from the extracellular matrix to the nucleus via the cytoskeleton may also directly contribute to the cell's ability to probe its mechanical environment by triggering force-induced changes in nuclear structures. In addition, it is now emerging that the physical properties of the nucleus play a crucial role during cell migration in three-dimensional (3D) environments, where cells often have to transit through narrow constrictions that are smaller than the nuclear diameter, e.g., during development, wound healing, or cancer metastasis. In this review, we provide a brief overview of how LINC complex proteins and lamins facilitate nucleo-cytoskeletal coupling, highlight recent findings regarding the role of the nucleus in cellular mechanotransduction and cell motility in 3D environments, and discuss how mutations and/or changes in the expression of these nuclear envelope proteins can result in a broad range of human diseases, including muscular dystrophy, dilated cardiomyopathy, and premature aging.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
