α1-antitrypsin deficiency and the hepatocytes - an elegans solution to drug discovery
- PMID: 24355812
- PMCID: PMC3970812
- DOI: 10.1016/j.biocel.2013.12.006
α1-antitrypsin deficiency and the hepatocytes - an elegans solution to drug discovery
Abstract
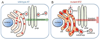
Hepatocytes are metabolically active cells of the liver that play an important role in the biosynthesis of proteins including α1-antitrypsin. Mutations in the α1-antitrypsin gene can lead to protein misfolding, polymerization/aggregation and retention of protein within the endoplasmic reticulum of hepatocytes. The intracellular accumulation of α1-antitrypsin aggregates can lead to liver disease and increased likelihood of developing hepatocellular carcinomas. Of note, only ~10% of individuals with α1-antitrypsin-deficiency develop severe liver disease suggesting that there are other genetic and/or environmental factors that determine disease outcome. The nematode, Caenorhabditis elegans, is a powerful genetic model organism to study molecular aspects of human disease. In this review, we discuss the functional similarities between the intestinal cells of C. elegans and human hepatocytes and how a C. elegans model of α1-antitrypsin-deficiency can be used as a tool for identifying genetic modifiers and small molecule drugs.
Keywords: C. elegans; Hepatocyte; Liver disease; Protein aggregation; α1-Antitrypsin deficiency.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
References
-
- Branicky R, Desjardins D, Liu JL, Hekimi S. Lipid transport and signaling in Caenorhabditis elegans. Dev Dyn. 2010;239:1365–1377. - PubMed
-
- Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci U S A. 2000;97:1796–17801. - PMC - PubMed
-
- Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency--a model for conformational diseases. N Engl J Med. 2002;346:45–53. - PubMed
-
- de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest. 2002;122:1818–1829. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

