Inflammatory pathways of seizure disorders
- PMID: 24355813
- PMCID: PMC3977596
- DOI: 10.1016/j.tins.2013.11.002
Inflammatory pathways of seizure disorders
Abstract
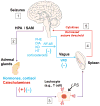
Epilepsy refers to a cluster of neurological diseases characterized by seizures. Although many forms of epilepsy have a well-defined immune etiology, in other forms of epilepsy an altered immune response is only suspected. In general, the hypothesis that inflammation contributes to seizures is supported by experimental results. Additionally, antiepileptic maneuvers may act as immunomodulators and anti-inflammatory therapies can treat seizures. Triggers of seizure include a bidirectional communication between the nervous system and organs of immunity. Thus, a crucial cellular interface protecting from immunological seizures is the blood-brain barrier (BBB). Here, we summarize recent advances in the understanding and treatment of epileptic seizures that derive from a non-neurocentric viewpoint and suggest key avenues for future research.
Keywords: antiepileptic drugs; blood–brain barrier; corticosteroids; immunomodulatory axis; infection; inflammation; vagus nerve stimulation.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Panayiotopoulos CP. The new ILAE report on terminology and concepts for the organization of epilepsies: critical review and contribution. Epilepsia. 2012;53:399–404. - PubMed
-
- Vezzani A, et al. Inflammation and epilepsy. Handb Clin Neurol. 2012;107:163–175. - PubMed
-
- Vezzani A, Janigro D. Inflammation. In: Engel G, Pedley TA, editors. Epilepsy: A comprehensive Textbook. Second. Lippincott Williams and Wilkins; 2008. pp. 267–276.
-
- Traynelis SF, Dingledine R. Potassium-induced spontaneous electrographic seizures in rat hippocampal slices. J Neurophysiol. 1988;59:259–276. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 HD057256/HD/NICHD NIH HHS/United States
- R01NS43284/NS/NINDS NIH HHS/United States
- R01 NS078307/NS/NINDS NIH HHS/United States
- R01NS078307/NS/NINDS NIH HHS/United States
- R21 NS077236/NS/NINDS NIH HHS/United States
- R01 NS043284/NS/NINDS NIH HHS/United States
- R42 MH093302/MH/NIMH NIH HHS/United States
- R42MH093302/MH/NIMH NIH HHS/United States
- R41MH093302/MH/NIMH NIH HHS/United States
- R21NS077236/NS/NINDS NIH HHS/United States
- R21HD057256/HD/NICHD NIH HHS/United States
- UH2 TR000491/TR/NCATS NIH HHS/United States
- UH3 TR000491/TR/NCATS NIH HHS/United States
- R41 MH093302/MH/NIMH NIH HHS/United States
- UH2TR000491/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

