Antisense oligonucleotide treatment ameliorates alpha-1 antitrypsin-related liver disease in mice
- PMID: 24355919
- PMCID: PMC3871221
- DOI: 10.1172/JCI67968
Antisense oligonucleotide treatment ameliorates alpha-1 antitrypsin-related liver disease in mice
Abstract
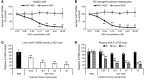
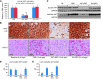
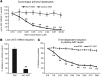
Alpha-1 antitrypsin deficiency (AATD) is a rare genetic disease that results from mutations in the alpha-1 antitrypsin (AAT) gene. The mutant AAT protein aggregates and accumulates in the liver leading to AATD liver disease, which is only treatable by liver transplant. The PiZ transgenic mouse strain expresses a human AAT (hAAT) transgene that contains the AATD-associated Glu342Lys mutation. PiZ mice exhibit many AATD symptoms, including AAT protein aggregates, increased hepatocyte death, and liver fibrosis. In the present study, we systemically treated PiZ mice with an antisense oligonucleotide targeted against hAAT (AAT-ASO) and found reductions in circulating levels of AAT and both soluble and aggregated AAT protein in the liver. Furthermore, AAT-ASO administration in these animals stopped liver disease progression after short-term treatment, reversed liver disease after long-term treatment, and prevented liver disease in young animals. Additionally, antisense oligonucleotide treatment markedly decreased liver fibrosis in this mouse model. Administration of AAT-ASO in nonhuman primates led to an approximately 80% reduction in levels of circulating normal AAT, demonstrating potential for this approach in higher species. Antisense oligonucleotides thus represent a promising therapy for AATD liver disease.
Figures



Similar articles
-
Knockdown of Z Mutant Alpha-1 Antitrypsin In Vivo Using Modified DNA Antisense Oligonucleotides.Methods Mol Biol. 2017;1639:127-138. doi: 10.1007/978-1-4939-7163-3_12. Methods Mol Biol. 2017. PMID: 28752452
-
Survival Advantage of Both Human Hepatocyte Xenografts and Genome-Edited Hepatocytes for Treatment of α-1 Antitrypsin Deficiency.Mol Ther. 2017 Nov 1;25(11):2477-2489. doi: 10.1016/j.ymthe.2017.09.020. Epub 2017 Sep 25. Mol Ther. 2017. PMID: 29032169 Free PMC article.
-
Amelioration of Alpha-1 Antitrypsin Deficiency Diseases with Genome Editing in Transgenic Mice.Hum Gene Ther. 2018 Aug;29(8):861-873. doi: 10.1089/hum.2017.227. Epub 2018 Jun 22. Hum Gene Ther. 2018. PMID: 29641323
-
Liver disease in alpha-1 antitrypsin deficiency: current understanding and future therapy.COPD. 2013 Mar;10 Suppl 1:35-43. doi: 10.3109/15412555.2013.765839. COPD. 2013. PMID: 23527737 Review.
-
RNAi therapeutics for diseases involving protein aggregation: fazirsiran for alpha-1 antitrypsin deficiency-associated liver disease.Expert Opin Investig Drugs. 2023 Jul-Dec;32(7):571-581. doi: 10.1080/13543784.2023.2239707. Epub 2023 Jul 24. Expert Opin Investig Drugs. 2023. PMID: 37470509 Review.
Cited by
-
Alpha-1 antitrypsin deficiency liver disease.Transl Gastroenterol Hepatol. 2021 Apr 5;6:23. doi: 10.21037/tgh.2020.02.23. eCollection 2021. Transl Gastroenterol Hepatol. 2021. PMID: 33824927 Free PMC article. Review.
-
A Compact Base Editor Rescues AATD-associated Liver and Lung Disease in Mouse Models.bioRxiv [Preprint]. 2025 May 9:2025.05.07.652636. doi: 10.1101/2025.05.07.652636. bioRxiv. 2025. PMID: 40654892 Free PMC article. Preprint.
-
Alpha-1-Antitrypsin Deficiency.Clin Liver Dis (Hoboken). 2022 Mar 27;19(3):89-92. doi: 10.1002/cld.1147. eCollection 2022 Mar. Clin Liver Dis (Hoboken). 2022. PMID: 35355837 Free PMC article. Review.
-
Exosome-associated miRNA-99a-5p targeting BMPR2 promotes hepatocyte apoptosis during the process of hepatic fibrosis.Clin Exp Med. 2023 Nov;23(7):4021-4031. doi: 10.1007/s10238-023-01122-0. Epub 2023 Jun 24. Clin Exp Med. 2023. PMID: 37354366
-
Don't Miss the BoAAT: Correctly Diagnosing Acute-on-Chronic Liver Disease.Dig Dis Sci. 2019 Oct;64(10):2780-2783. doi: 10.1007/s10620-019-05816-y. Dig Dis Sci. 2019. PMID: 31456092 No abstract available.
References
-
- Janciauskiene SM, Bals R, Koczulla R, Vogelmeier C, Kohnlein T, Welte T. The discovery of alpha1-antitrypsin and its role in health and disease. Respir Med. 2011;105(8):1129–1139. - PubMed
-
- Ekeowa UI, et al. α1-Antitrypsin deficiency, chronic obstructive pulmonary disease and the serpinopathies. Clin Sci (Lond). 2009;116(12):837–850. - PubMed
-
- Greene CM, et al. α-1 Antitrypsin deficiency: a conformational disease associated with lung and liver manifestations. J Inherit Metab Dis. 2008;31(1):21–34. - PubMed
-
- Bals R. α-1-Antitrypsin deficiency. Best Pract Res Clin Gastroenterol. 2010;24(5):629–633. - PubMed
-
- Teckman JH, Lindblad D. α-1-Antitrypsin deficiency: diagnosis, pathophysiology, and management. Curr Gastroenterol Rep. 2006;8(1):14–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

