Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir
- PMID: 24355920
- PMCID: PMC3871222
- DOI: 10.1172/JCI68730
Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir
Abstract
Background: The majority of patients receiving the platinum-based chemotherapy drug oxaliplatin develop peripheral neurotoxicity. Because this neurotoxicity involves ROS production, we investigated the efficacy of mangafodipir, a molecule that has antioxidant properties and is approved for use as an MRI contrast enhancer.
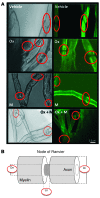
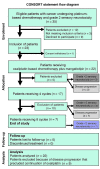
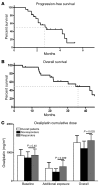
Methods: The effects of mangafodipir were examined in mice following treatment with oxaliplatin. Neurotoxicity, axon myelination, and advanced oxidized protein products (AOPPs) were monitored. In addition, we enrolled 23 cancer patients with grade ≥ 2 oxaliplatin-induced neuropathy in a phase II study, with 22 patients receiving i.v. mangafodipir following oxaliplatin. Neuropathic effects were monitored for up to 8 cycles of oxaliplatin and mangafodipir.
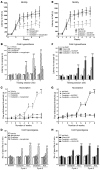
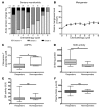
Results: Mangafodipir prevented motor and sensory dysfunction and demyelinating lesion formation. In mice, serum AOPPs decreased after 4 weeks of mangafodipir treatment. In 77% of patients treated with oxaliplatin and mangafodipir, neuropathy improved or stabilized after 4 cycles. After 8 cycles, neurotoxicity was downgraded to grade ≥ 2 in 6 of 7 patients. Prior to enrollment, patients received an average of 880 ± 239 mg/m2 oxaliplatin. Patients treated with mangafodipir tolerated an additional dose of 458 ± 207 mg/m2 oxaliplatin despite preexisting neuropathy. Mangafodipir responders managed a cumulative dose of 1,426 ± 204 mg/m2 oxaliplatin. Serum AOPPs were lower in responders compared with those in nonresponders.
Conclusion: Our study suggests that mangafodipir can prevent and/or relieve oxaliplatin-induced neuropathy in cancer patients. Trial registration. Clinicaltrials.gov NCT00727922. Funding. Université Paris Descartes, Ministère de la Recherche et de l'Enseignement Supérieur, and Assistance Publique-Hôpitaux de Paris.
Figures
Comment in
-
The search for treatments to reduce chemotherapy-induced peripheral neuropathy.J Clin Invest. 2014 Jan;124(1):72-4. doi: 10.1172/JCI73908. Epub 2013 Dec 20. J Clin Invest. 2014. PMID: 24355918 Free PMC article.
References
-
- Laurent A, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65(3):948–956. - PubMed
-
- Gamelin E, Gamelin L, Bossi L, Quasthoff S. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measures. Semin Oncol. 2002;29(5 suppl 15):21–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

