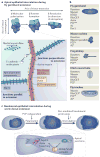
Cell intercalation from top to bottom
- PMID: 24355988
- PMCID: PMC4550482
- DOI: 10.1038/nrm3723
Cell intercalation from top to bottom
Abstract
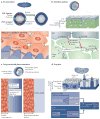
Animal development requires a carefully orchestrated cascade of cell fate specification events and cellular movements. A surprisingly small number of choreographed cellular behaviours are used repeatedly to shape the animal body plan. Among these, cell intercalation lengthens or spreads a tissue at the expense of narrowing along an orthogonal axis. Key steps in the polarization of both mediolaterally and radially intercalating cells have now been clarified. In these different contexts, intercalation seems to require a distinct combination of mechanisms, including adhesive changes that allow cells to rearrange, cytoskeletal events through which cells exert the forces needed for cell neighbour exchange, and in some cases the regulation of these processes through planar cell polarity.
Conflict of interest statement
Figures


References
Works Cited
-
- Keller R. Developmental biology. Physical biology returns to morphogenesis. Science. 2012;338:201–3. - PubMed
-
- Keller R. Mechanisms of elongation in embryogenesis. Development. 2006;133:2291–2302. - PubMed
-
- Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–53. - PubMed
-
- Tada M, Kai M. Planar cell polarity in coordinated and directed movements. Curr Top Dev Biol. 2012;101:77–110. - PubMed
Highlighted References
-
- Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

