Branch management: mechanisms of axon branching in the developing vertebrate CNS
- PMID: 24356070
- PMCID: PMC4063290
- DOI: 10.1038/nrn3650
Branch management: mechanisms of axon branching in the developing vertebrate CNS
Abstract
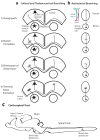
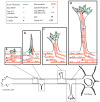
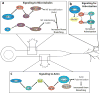
The remarkable ability of a single axon to extend multiple branches and form terminal arbors enables vertebrate neurons to integrate information from divergent regions of the nervous system. Axons select appropriate pathways during development, but it is the branches that extend interstitially from the axon shaft and arborize at specific targets that are responsible for virtually all of the synaptic connectivity in the vertebrate CNS. How do axons form branches at specific target regions? Recent studies have identified molecular cues that activate intracellular signalling pathways in axons and mediate dynamic reorganization of the cytoskeleton to promote the formation of axon branches.
Figures

References
-
- Acebes A, Ferrus A. Cellular and molecular features of axon collaterals and dendrites. Trends Neurosci. 2000;23:557–65. - PubMed
-
- Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol. 2011;71:201–20. - PubMed
-
- Kornack DR, Giger RJ. Probing microtubule +TIPs: regulation of axon branching. Curr Opin Neurobiol. 2005;15:58–66. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

