Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study
- PMID: 24356593
- PMCID: PMC3867989
- DOI: 10.2337/dc13-2251
Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study
Abstract
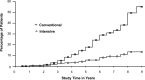
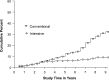
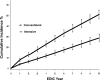
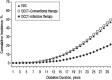
OBJECTIVE To evaluate whether intensive treatment (INT) with the goal of achieving blood glucose levels as close to the nondiabetic range as safely possible reduced the risk of onset and progression of diabetic retinopathy (DR) in subjects with type 1 diabetes (T1D) compared with conventional therapy (CON). RESEARCH DESIGN AND METHODS The Diabetes Control and Complications Trial (DCCT) (1982-1993) was a multicenter, controlled clinical trial comparing INT with CON for onset and progression of DR. The Epidemiology of Diabetes Interventions and Complications (EDIC) study (1994-present) is an observational follow-up of the DCCT cohort. RESULTS Of the 1,441 DCCT subjects, 726 had no DR (primary prevention cohort) and 715 had mild DR (secondary intervention cohort) at baseline. Subjects were followed for a mean of 6.5 years. INT median HbA1c was 7% compared with CON median of 9%. INT reduced the adjusted mean risk for the development of DR by 76% and slowed progression of DR by 54% compared with CON. Following DCCT, the HbA1c levels in the original INT and CON groups converged (year 8, INT 7.98%; CON 8.07%); nevertheless, the groups continued to have a durable effect of initial assigned therapy with significantly lower incidence of further DR progression in the INT group (hazard reduction 53-56%). Severe retinal outcomes and procedures to treat them were reduced by 50% in the original INT group. CONCLUSIONS INT delays the onset and slows the progression of DR. Furthermore, the early effects of metabolic control continue to accrue over many years despite subsequent comparable glycemic control (metabolic memory). These results emphasize the need for optimizing glycemic control as early as possible in patients with diabetes.
Figures


References
-
- Joslin EP. The Treatment of Diabetes Mellitus. Philadelphia, PA, and New York, NY, Lea and Febiger, 1916
-
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
-
- The DCCT Research Group The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 - PubMed
-
- Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl):823–833 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

