Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study
- PMID: 24356594
- PMCID: PMC3867994
- DOI: 10.2337/dc13-2113
Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study
Abstract
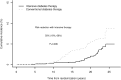
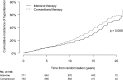
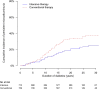
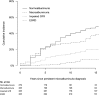
OBJECTIVE Kidney disease manifests clinically as elevated albumin excretion rate (AER), impaired glomerular filtration rate (GFR), or both, and is a cause of substantial morbidity and mortality in type 1 diabetes (T1D). The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study tested whether intensive diabetes therapy (INT) aimed at lowering glucose concentrations as close as safely possible to the normal range reduces the risks of kidney disease and other diabetes complications. RESEARCH DESIGN AND METHODS In the DCCT, 1,441 participants with T1D were randomly assigned to INT or conventional diabetes therapy (CON) for a mean duration of 6.5 years. Subsequently, participants have been followed for 18 years in the ongoing observational EDIC. Standardized longitudinal measurements of AER, estimated GFR, and blood pressure were made throughout the DCCT/EDIC. RESULTS During the DCCT, INT reduced the risks of incident microalbuminuria (AER ≥40 mg/24 h) and macroalbuminuria (AER ≥300 mg/24 h) by 39% (95% CI 21-52%) and 54% (29-74%), respectively. During EDIC years 1-8, participants previously assigned to DCCT INT continued to experience lower rates of incident microalbuminuria and macroalbuminuria, with risk reductions of 59% (39-73%) and 84% (67-92%), respectively. Beneficial effects of INT on the development of impaired GFR (sustained estimated GFR <60 mL/min/1.73 m(2)) and hypertension became evident during combined DCCT/EDIC follow-up, with risk reductions of 50% (18-69%) and 20% (6-21%), respectively, compared with CON. CONCLUSIONS In the DCCT/EDIC, INT resulted in clinically important, durable reductions in the risks of microalbuminuria, macroalbuminuria, impaired GFR, and hypertension.
Figures
References
-
- Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 1983;25:496–501 - PubMed
-
- Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785–794 - PubMed
-
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK087726/DK/NIDDK NIH HHS/United States
- U01-DK-094157/DK/NIDDK NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- U01 DK094157/DK/NIDDK NIH HHS/United States
- U01-DK-094176/DK/NIDDK NIH HHS/United States
- R01-DK-088762/DK/NIDDK NIH HHS/United States
- R01-DK-087726/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- U01 DK094176/DK/NIDDK NIH HHS/United States
- R01 DK088762/DK/NIDDK NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- UL1 TR000114/TR/NCATS NIH HHS/United States
- RC4 DK090766/DK/NIDDK NIH HHS/United States
- RC4-DK-090766/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

