Cancer-associated CD43 glycoforms as target of immunotherapy
- PMID: 24356816
- PMCID: PMC3954431
- DOI: 10.1158/1535-7163.MCT-13-0651
Cancer-associated CD43 glycoforms as target of immunotherapy
Abstract
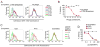
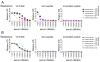
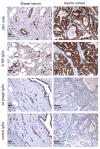
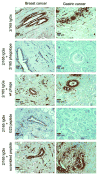
CD43 is a sialoglycosylated membrane protein that is involved in cell proliferation and differentiation. CD43 glycoforms that are recognized by the UN1 monoclonal antibody (mAb) were expressed in lymphoblastoid T-cell lines and solid tumors, such as breast, colon, gastric, and squamous cell lung carcinomas, while unexpressed in the normal counterparts. The cancer association of UN1/CD43 epitope suggested the possibility to use the UN1 mAb for tumor diagnosis and therapy. In this study, we show that the UN1 mAb was endowed with antitumor activity in vivo because its passive transfer inhibited the growth of UN1-positive HPB-ALL lymphoblastoid T cells in mice. Furthermore, we demonstrate that tumor inhibition was due to UN1 mAb-dependent natural killer-mediated cytotoxicity. By screening a phage-displayed random peptide library, we identified the phagotope 2/165 as a mimotope of the UN1 antigen, as it harbored a peptide sequence that was specifically recognized by the UN1 mAb and inhibited the binding of the UN1 mAb to UN1-positive tumor cells. On the basis of sequence homology with the extracellular region of CD43 (amino acids 64 to 83), the 2/165 peptide sequence was likely mimicking the protein core of the UN1/CD43 epitope. When used as vaccine in mice, the 2/165 phagotope raised antibodies against the UN1/CD43 antigen, indicating that the 2/165 phagotope mimicked the UN1 antigen structure, and could represent a novel immunogen for cancer immunotherapy. These findings support the feasibility of using monoclonal antibodies to identify cancer-associated mimotopes for immunotherapy.
©2013 AACR.
Conflict of interest statement
Figures


Similar articles
-
Mass spectrometry-based identification of the tumor antigen UN1 as the transmembrane CD43 sialoglycoprotein.Mol Cell Proteomics. 2011 May;10(5):M111.007898. doi: 10.1074/mcp.M111.007898. Epub 2011 Mar 3. Mol Cell Proteomics. 2011. PMID: 21372249 Free PMC article.
-
UN1, a murine monoclonal antibody recognizing a novel human thymic antigen.Tissue Antigens. 1994 Aug;44(2):73-82. doi: 10.1111/j.1399-0039.1994.tb02362.x. Tissue Antigens. 1994. PMID: 7817381
-
Partial purification and MALDI-TOF MS analysis of UN1, a tumor antigen membrane glycoprotein.Int J Biol Macromol. 2006 Aug 15;39(1-3):122-6. doi: 10.1016/j.ijbiomac.2006.02.020. Epub 2006 Apr 3. Int J Biol Macromol. 2006. PMID: 16580720
-
Aberrant glycosylation as biomarker for cancer: focus on CD43.Biomed Res Int. 2014;2014:742831. doi: 10.1155/2014/742831. Epub 2014 Feb 13. Biomed Res Int. 2014. PMID: 24689054 Free PMC article. Review.
-
Mimotope vaccination--from allergy to cancer.Expert Opin Biol Ther. 2009 Apr;9(4):493-506. doi: 10.1517/14712590902870386. Expert Opin Biol Ther. 2009. PMID: 19344285 Free PMC article. Review.
Cited by
-
Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression.Int J Mol Sci. 2020 Aug 20;21(17):5983. doi: 10.3390/ijms21175983. Int J Mol Sci. 2020. PMID: 32825245 Free PMC article. Review.
-
Targeting CD43 optimizes cancer immunotherapy through reinvigorating antitumor immune response in colorectal cancer.Cell Oncol (Dordr). 2023 Jun;46(3):777-791. doi: 10.1007/s13402-023-00794-w. Epub 2023 Mar 15. Cell Oncol (Dordr). 2023. PMID: 36920728
-
Genome-wide CRISPR screens reveal a specific ligand for the glycan-binding immune checkpoint receptor Siglec-7.Proc Natl Acad Sci U S A. 2021 Feb 2;118(5):e2015024118. doi: 10.1073/pnas.2015024118. Proc Natl Acad Sci U S A. 2021. PMID: 33495350 Free PMC article.
-
Differential Expression of CD43, CD81, and CD200 in Classic Versus Variant Hairy Cell Leukemia.Cytometry B Clin Cytom. 2019 Jul;96(4):275-282. doi: 10.1002/cyto.b.21785. Epub 2019 May 11. Cytometry B Clin Cytom. 2019. PMID: 31077558 Free PMC article.
-
OncodriveFML: a general framework to identify coding and non-coding regions with cancer driver mutations.Genome Biol. 2016 Jun 16;17(1):128. doi: 10.1186/s13059-016-0994-0. Genome Biol. 2016. PMID: 27311963 Free PMC article.
References
-
- Kadaja-Saarepuu L, Looke M, Balikova A, Maimets T. Tumor suppressor p53 down-regulates expression of human leukocyte marker CD43 in non-hematopoietic tumor cells. International journal of oncology. 2012;40:567–76. - PubMed
-
- Ostberg JR, Barth RK, Frelinger JG. The Roman god Janus: a paradigm for the function of CD43. Immunology today. 1998;19:546–50. - PubMed
-
- Tassone P, Bond H, Bonelli P, Tuccillo F, Valerio G, Petrella A, et al. UN1, a murine monoclonal antibody recognizing a novel human thymic antigen. Tissue antigens. 1994;44:73–82. - PubMed
-
- Cecco L, Bond HM, Bonelli P, Tuccillo F, Cerra M, Tassone P, et al. Purification and characterization of a human sialoglycoprotein antigen expressed in immature thymocytes and fetal tissues. Tissue antigens. 1998;51:528–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

