Human uterine and placental arteries exhibit tissue-specific acute responses to 17β-estradiol and estrogen-receptor-specific agonists
- PMID: 24356876
- PMCID: PMC4004081
- DOI: 10.1093/molehr/gat095
Human uterine and placental arteries exhibit tissue-specific acute responses to 17β-estradiol and estrogen-receptor-specific agonists
Abstract
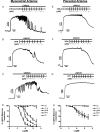
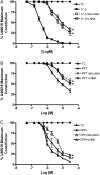
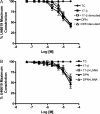
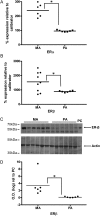
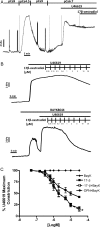
The discrete regulation of vascular tone in the human uterine and placental circulations is a key determinant of appropriate uteroplacental blood perfusion and pregnancy success. Humoral factors such as estrogen, which increases in the placenta and maternal circulation throughout human pregnancy, may regulate these vascular beds as studies of animal arteries have shown that 17β-estradiol, or agonists of estrogen receptors (ER), can exert acute vasodilatory actions. The aim of this study was to compare how acute exposure to ER-specific agonists, and 17β-estradiol, altered human placental and uterine arterial tone in vitro. Uterine and placental arteries were isolated from biopsies obtained from women with uncomplicated pregnancy delivering a singleton infant at term. Vessels were mounted on a wire myograph, exposed to the thromboxane receptor agonist U46619 (10(-6) M), and then incubated with incremental doses (5 min, 0.03-30 µM) of either 17β-estradiol or agonists specific for the ERs ERα (PPT), ERβ (DPN) or the G-protein-coupled estrogen receptor GPER-1 (G1). ERα and ERβ mRNA expression was assessed. 17β-estradiol, PPT and DPN each relaxed myometrial arteries (P < 0.05) in a manner that was partly endothelium-dependent. In contrast, 17β-estradiol or DPN relaxed placental arteries (maximum relaxation to 42 ± 1.1 or 47.6 ± 6.53% of preconstriction, respectively) to a lesser extent than myometrial arteries (to 0.03 ± 0.03 or 8.0 ± 1.0%) and in an endothelial-independent manner whereas PPT was without effect. G1 exposure did not inhibit the constriction of myometrial nor placenta arteries. mRNA expression of ERα and ERβ was greater in myometrial arteries than placental arteries. ER-specific agonists, and 17β-estradiol, differentially modulate the tone of uterine versus placental arteries highlighting that estrogen may regulate human uteroplacental blood flow in a tissue-specific manner.
Keywords: ER agonists; estrogen; human placental arteries; human uterine arteries; vascular tone.
Figures
References
-
- Al Zubair K, Razak A, Bexis S, Docherty JR. Relaxations to oestrogen receptor subtype selective agonists in rat and mouse arteries. Eur J Pharmacol. 2005;513:101–108. - PubMed
-
- Bolego C, Cignarella A, Sanvito P, Pelosi V, Pellegatta F, Puglisis L, Pinna C. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-α agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther. 2005;313:1203–1208. - PubMed
-
- Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. - PubMed
-
- Corcoran JJ, Charnock JC, Martin J, Taggart MJ, Westwood M. Differential effect of insulin like growth factor-I on constriction of human uterine and placental arteries. J Clin Endocrinol Metab. 2012;97:e2098–e2104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

