Characterization of the deubiquitinating activity of USP19 and its role in endoplasmic reticulum-associated degradation
- PMID: 24356957
- PMCID: PMC3916552
- DOI: 10.1074/jbc.M113.538934
Characterization of the deubiquitinating activity of USP19 and its role in endoplasmic reticulum-associated degradation
Abstract
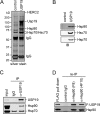
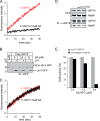
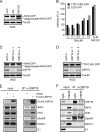
Deubiquitinating enzymes (DUBs) regulate various cellular processes ranging from protein degradation to cellular signaling. USP19, the only DUB containing a carboxyl-terminal transmembrane domain, was proposed to function in endoplasmic reticulum-associated degradation (ERAD). Here we characterize the function and regulation of USP19. We identify Hsp90 as a specific partner that binds the catalytic domain of USP19 to promote substrate association. Intriguingly, although overexpressed USP19 interacts with Derlin-1 and other ERAD machinery factors in the membrane, endogenous USP19 is mostly in the cytosol where it binds Hsp90. Accordingly, we detect neither interaction of endogenous USP19 with Derlin-1 nor significant effect on ERAD by USP19 depletion. The USP19 transmembrane domain appears to be partially stabilized in the cytosol by an interaction with its own catalytic domain, resulting in auto-inhibition of its deubiquitinating activity. These results clarify the role of USP19 in ERAD and suggest a novel DUB regulation that involves chaperone association and membrane integration. Moreover, our study indicates that the localization of tail-anchored membrane proteins can be subject to regulation in cells.
Keywords: Deubiquitination; ER-associated Degradation; Hsp90; Membrane Proteins; Tail-anchored Protein Biogenesis; USP19; Ubiquitin.
Figures



Similar articles
-
USP19-Mediated Deubiquitination Facilitates the Stabilization of HRD1 Ubiquitin Ligase.Int J Mol Sci. 2016 Nov 2;17(11):1829. doi: 10.3390/ijms17111829. Int J Mol Sci. 2016. PMID: 27827840 Free PMC article.
-
Ubiquitin-specific protease 19 regulates the stability of the E3 ubiquitin ligase MARCH6.Exp Cell Res. 2014 Oct 15;328(1):207-216. doi: 10.1016/j.yexcr.2014.07.025. Epub 2014 Aug 1. Exp Cell Res. 2014. PMID: 25088257
-
Domain interactions reveal auto-inhibition of the deubiquitinating enzyme USP19 and its activation by HSP90 in the modulation of huntingtin aggregation.Biochem J. 2020 Nov 13;477(21):4295-4312. doi: 10.1042/BCJ20200536. Biochem J. 2020. PMID: 33094816
-
The evolving role of ubiquitin modification in endoplasmic reticulum-associated degradation.Biochem J. 2017 Feb 15;474(4):445-469. doi: 10.1042/BCJ20160582. Biochem J. 2017. PMID: 28159894 Free PMC article. Review.
-
Membrane Protein Quantity Control at the Endoplasmic Reticulum.J Membr Biol. 2017 Aug;250(4):379-392. doi: 10.1007/s00232-016-9931-0. Epub 2016 Oct 14. J Membr Biol. 2017. PMID: 27743014 Free PMC article. Review.
Cited by
-
Leucine modulates the IPEC-J2 cell proteome associated with cell proliferation, metabolism and phagocytosis.Anim Nutr. 2018 Sep;4(3):316-321. doi: 10.1016/j.aninu.2018.03.006. Epub 2018 Apr 5. Anim Nutr. 2018. PMID: 30175261 Free PMC article.
-
Deubiquitinases in cancer.Oncotarget. 2015 May 30;6(15):12872-89. doi: 10.18632/oncotarget.3671. Oncotarget. 2015. PMID: 25972356 Free PMC article. Review.
-
Deubiquitinases USP20/33 promote the biogenesis of tail-anchored membrane proteins.J Cell Biol. 2021 May 3;220(5):e202004086. doi: 10.1083/jcb.202004086. J Cell Biol. 2021. PMID: 33792613 Free PMC article.
-
USP19 deubiquitinating enzyme inhibits muscle cell differentiation by suppressing unfolded-protein response signaling.Mol Biol Cell. 2015 Mar 1;26(5):913-23. doi: 10.1091/mbc.E14-06-1129. Epub 2015 Jan 7. Mol Biol Cell. 2015. PMID: 25568336 Free PMC article.
-
DNAJC5 facilitates USP19-dependent unconventional secretion of misfolded cytosolic proteins.Cell Discov. 2018 Mar 6;4:11. doi: 10.1038/s41421-018-0012-7. eCollection 2018. Cell Discov. 2018. PMID: 29531792 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

