The fibrinogen-binding M1 protein reduces pharyngeal cell adherence and colonization phenotypes of M1T1 group A Streptococcus
- PMID: 24356958
- PMCID: PMC3916555
- DOI: 10.1074/jbc.M113.529537
The fibrinogen-binding M1 protein reduces pharyngeal cell adherence and colonization phenotypes of M1T1 group A Streptococcus
Abstract
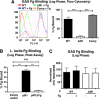
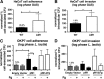
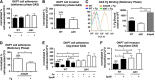
Group A Streptococcus (GAS) is a leading human pathogen producing a diverse array of infections from simple pharyngitis ("strep throat") to invasive conditions, including necrotizing fasciitis and toxic shock syndrome. The surface-anchored GAS M1 protein is a classical virulence factor that promotes phagocyte resistance and exaggerated inflammation by binding host fibrinogen (Fg) to form supramolecular networks. In this study, we used a virulent WT M1T1 GAS strain and its isogenic M1-deficient mutant to examine the role of M1-Fg binding in a proximal step in GAS infection-interaction with the pharyngeal epithelium. Expression of the M1 protein reduced GAS adherence to human pharyngeal keratinocytes by 2-fold, and this difference was increased to 4-fold in the presence of Fg. In stationary phase, surface M1 protein cleavage by the GAS cysteine protease SpeB eliminated Fg binding and relieved its inhibitory effect on GAS pharyngeal cell adherence. In a mouse model of GAS colonization of nasal-associated lymphoid tissue, M1 protein expression was associated with an average 6-fold decreased GAS recovery in isogenic strain competition assays. Thus, GAS M1 protein-Fg binding reduces GAS pharyngeal cell adherence and colonization in a fashion that is counterbalanced by SpeB. Inactivation of SpeB during the shift to invasive GAS disease allows M1-Fg binding, increasing pathogen phagocyte resistance and proinflammatory activities.
Keywords: Bacterial Adhesion; Bacterial Pathogenesis; Fibrinogen; Streptococcus pyogenes; Virulence Factors.
Figures


References
-
- Cole J. N., Barnett T. C., Nizet V., Walker M. J. (2011) Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 9, 724–736 - PubMed
-
- Carapetis J. R., Steer A. C., Mulholland E. K., Weber M. (2005) The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5, 685–694 - PubMed
-
- Young M. H., Aronoff D. M., Engleberg N. C. (2005) Necrotizing fasciitis: pathogenesis and treatment. Expert Rev. Anti-Infect. Ther. 3, 279–294 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

