Development of the mechanical properties of engineered skin substitutes after grafting to full-thickness wounds
- PMID: 24356985
- PMCID: PMC4023834
- DOI: 10.1115/1.4026290
Development of the mechanical properties of engineered skin substitutes after grafting to full-thickness wounds
Abstract
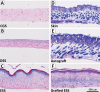
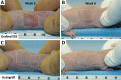
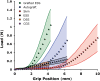
Engineered skin substitutes (ESSs) have been reported to close full-thickness burn wounds but are subject to loss from mechanical shear due to their deficiencies in tensile strength and elasticity. Hypothetically, if the mechanical properties of ESS matched those of native skin, losses due to shear or fracture could be reduced. To consider modifications of the composition of ESS to improve homology with native skin, biomechanical analyses of the current composition of ESS were performed. ESSs consist of a degradable biopolymer scaffold of type I collagen and chondroitin-sulfate (CGS) that is populated sequentially with cultured human dermal fibroblasts (hF) and epidermal keratinocytes (hK). In the current study, the hydrated biopolymer scaffold (CGS), the scaffold populated with hF dermal skin substitute (DSS), or the complete ESS were evaluated mechanically for linear stiffness (N/mm), ultimate tensile load at failure (N), maximum extension at failure (mm), and energy absorbed up to the point of failure (N-mm). These biomechanical end points were also used to evaluate ESS at six weeks after grafting to full-thickness skin wounds in athymic mice and compared to murine autograft or excised murine skin. The data showed statistically significant differences (p <0.05) between ESS in vitro and after grafting for all four structural properties. Grafted ESS differed statistically from murine autograft with respect to maximum extension at failure, and from intact murine skin with respect to linear stiffness and maximum extension. These results demonstrate rapid changes in mechanical properties of ESS after grafting that are comparable to murine autograft. These values provide instruction for improvement of the biomechanical properties of ESS in vitro that may reduce clinical morbidity from graft loss.
Figures


References
-
- Boyce, S. T. , 2004, “Fabrication, Quality Assurance, and Assessment of Cultured Skin Substitutes for Treatment of Skin Wounds,” Biochem. Eng. J., 20(2), pp. 107–112, 2004 10.1016/j.bej.2003.09.017 - DOI
-
- American Burn Association, 2013. National Burn Repository, American Burn Association, Chicago, IL.
-
- Zeng, Q. , Macri, L. K. , Prasad, A. , Clark, R. A. F. , Zeugolis, D. I. , Hanley, C. , Garcia, Y. , and Pandit, A. , 2011, “Skin Tissue Engineering”, Comprehensive Biomaterials, Ducheyne P., Healy K., Hutmacher D. E., Grainger D. W., and Kirkpatrick C. J., eds., Elsevier, Philadelphia, PA, pp. 467–499.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

