Carboxyl terminus of ADAMTS13 directly inhibits platelet aggregation and ultra large von Willebrand factor string formation under flow in a free-thiol-dependent manner
- PMID: 24357063
- PMCID: PMC4013287
- DOI: 10.1161/ATVBAHA.113.302547
Carboxyl terminus of ADAMTS13 directly inhibits platelet aggregation and ultra large von Willebrand factor string formation under flow in a free-thiol-dependent manner
Abstract
Objective: ADAMTS13 (A Disintegrin And Metalloprotease with Thrombospondin type 1 repeats, 13) cleaves von Willebrand factor (VWF), thereby inhibiting thrombus formation. Proteolytic cleavage relies on the amino-terminal (MDTCS) domains, but the role of the more distal carboxyl-terminal domains of ADAMTS13 is not fully understood. A previous study demonstrated the presence of multiple surface-exposed free sulfhydryls on ADAMTS13 that seemed to interact with those on VWF under shear. Here, we determined the physiological relevance of such an interaction in antithrombotic responses under flow.
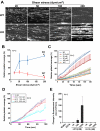
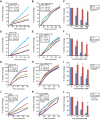
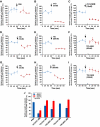
Approach and results: A microfluidic assay demonstrated that a carboxyl-terminal fragment of ADAMTS13, comprising either 2 to 8 thrombospondin type 1 (TSP1) repeats and CUB domains (T2C) or 5 to 8 Thrombospondin type 1 (TSP1) repeats and CUB domains (T5C), directly inhibited platelet adhesion/aggregation on a collagen surface under arterial shear. In addition, an intravital microscopic imaging analysis showed that the carboxyl-terminal fragment of ADAMTS13 (T2C or T5C) was capable of inhibiting the formation and elongation of platelet-decorated ultra large (UL) VWF strings and the adhesion of platelets/leukocytes on endothelium in mesenteric venules after oxidative injury. The inhibitory activity of T2C and T5C on platelet aggregation and ULVWF string formation were dependent on the presence of their surface free thiols; pretreatment of T2C and T5C or full-length ADAMTS13 with N-ethylmaleimide that reacts with free sulfhydryls abolished or significantly reduced its antithrombotic activity.
Conclusions: Our results demonstrate for the first time that the carboxyl terminus of ADAMTS13 has direct antithrombotic activity in a free-thiol-dependent manner. The free thiols in the carboxyl-terminal domains of ADAMTS13 may also contribute to the overall antithrombotic function of ADAMTS13 under pathophysiological conditions.
Keywords: ADAMTS13; animal model; arterial thrombosis; thrombotic thrombocytopenic purpura; von Willeband factor.
Figures



References
-
- Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. - PubMed
-
- Dong JF, Moake JL, Bernardo A, Fujikawa K, Ball C, Nolasco L, Lopez JA, Cruz MA. ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultra-large von Willebrand factor. J Biol Chem. 2003;278:29633–19639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

