A prospective analysis of co-processed non-ionic surfactants in enhancing permeability of a model hydrophilic drug
- PMID: 24357111
- PMCID: PMC3969477
- DOI: 10.1208/s12249-013-0065-8
A prospective analysis of co-processed non-ionic surfactants in enhancing permeability of a model hydrophilic drug
Abstract
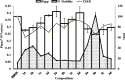
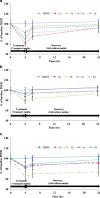
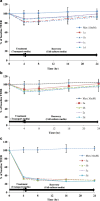
Paracellular route is a natural pathway for the transport of many hydrophilic drugs and macromolecules. The purpose of this study was to prospectively evaluate the ability of novel co-processed non-ionic surfactants to enhance the paracellular permeability of a model hydrophilic drug metformin using Caco-2 (human colonic adenocarcinoma) cell model. A three-tier screen was undertaken to evaluate the co-processed blends based on cytotoxicity, cellular integrity, and permeability coefficient. The relative contribution of the paracellular and the transcellular route in overall transport of metformin by co-processed blends was determined. Immunocytochemistry was conducted to determine the distribution of tight-junction protein claudin-1 after incubation with the co-processed blends. It was found that novel blends of Labrasol and Transcutol-P enhanced metformin permeability by approximately twofold with transient reduction in the transepithelia electrical resistance (TEER) and minimal cytotoxicity compared with the control, with the paracellular pathway as the major route of metformin transport. Maximum permeability of metformin (∼10-fold) was mediated by Tween-20 blends along with >75% reduction in the TEER which was irreversible over 24-h period. A shift in metformin transport from the paracellular to the transcellular route was observed with some Tween-20 blends. Immunocytochemical analysis revealed rearrangement of the cellular borders and fragmentation on treatment with Tween-20 blends. In conclusion, cytotoxicity, cellular integrity, and permeability of the hydrophilic drugs can be greatly influenced by the polyoxyethylene residues and medium chain fatty acids in the non-ionic surfactants at clinically relevant concentrations and therefore should be thoroughly investigated prior to their inclusion in formulations.
Figures


References
-
- Aungst BJ, Saitoh H, Burcham DL, Huang S, Mousa SA, Hussain MA. Enhancement of the intestinal absorption of peptides and nonpeptides. J Contr Rel. 1996;41(1–2):19–31. doi: 10.1016/0168-3659(96)01353-3. - DOI
-
- Balda M, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134(4):1031–49. doi: 10.1083/jcb.134.4.1031. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

