Endothelial nitric oxide: protector of a healthy mind
- PMID: 24357508
- PMCID: PMC3977136
- DOI: 10.1093/eurheartj/eht544
Endothelial nitric oxide: protector of a healthy mind
Abstract
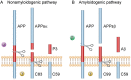
Endothelial nitric oxide (NO) is generated by constitutively active endothelial nitric oxide synthase (eNOS), an essential enzyme responsible for cardiovascular homeostasis. Historically, endothelial NO was first recognized as a major vasodilator involved in control of vasomotor function and local blood flow. In this review, our attention is focused on the emerging role of endothelial NO in linking cerebrovascular function with cognition. We will discuss the recognized ability of endothelial NO to modulate processing of amyloid precursor protein (APP), influence functional status of microglia, and affect cognitive function. Existing evidence suggests that the loss of NO in cultured human cerebrovascular endothelium causes increased expression of APP and β-site APP-cleaving enzyme 1 (BACE1) thereby resulting in increased secretion of amyloid β peptides (Aβ1-40 and Aβ1-42). Furthermore, increased expression of APP and BACE1 as well as increased production of Aβ peptides was detected in the cerebral microvasculature and brain tissue of eNOS-deficient mice. Since Aβ peptides are considered major cytotoxic molecules responsible for the pathogenesis of Alzheimer's disease, these observations support the concept that a loss of endothelial NO might significantly contribute to the initiation and progression of cognitive decline. In addition, genetic inactivation of eNOS causes activation of microglia and promotes a pro-inflammatory phenotype in the brain. Behavioural analysis revealed that eNOS-deficient mice exhibit impaired cognitive performance thereby indicating that selective loss of endothelial NO has a detrimental effect on the function of neuronal cells. Together with findings from prior studies demonstrating the ability of endothelial NO to affect synaptic plasticity, mitochondrial biogenesis, and function of neuronal progenitor cells, it is becoming apparent that the role of endothelial NO in the control of central nervous system function is very complex. We propose that endothelial NO represents the key molecule linking cerebrovascular and neuronal function.
Keywords: Alzheimer's disease; Amyloid precursor protein; BACE1; Cognitive impairment; Endothelium; Exercise; Hippocampus; Microglia; Mitochondria; Neuronal progenitors; Nitric oxide; Vascular dementia.
Figures

References
-
- Muqtadar H, Testai FD, Gorelick PB. The dementia of cardiac disease. Curr Cardiol Rep. 2012;14:732–740. - PubMed
-
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Leiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. - PubMed
-
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

