The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus
- PMID: 24357543
- PMCID: PMC3990679
- DOI: 10.1002/embj.201386609
The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus
Abstract
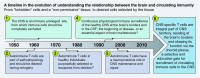
Inflammation is an integral part of the body's physiological repair mechanism, unless it remains unresolved and becomes pathological, as evident in the progressive nature of neurodegeneration. Based on studies from outside the central nervous system (CNS), it is now understood that the resolution of inflammation is an active process, which is dependent on well-orchestrated innate and adaptive immune responses. Due to the immunologically privileged status of the CNS, such resolution mechanism has been mostly ignored. Here, we discuss resolution of neuroinflammation as a process that depends on a network of immune cells operating in a tightly regulated sequence, involving the brain's choroid plexus (CP), a unique neuro-immunological interface, positioned to integrate signals it receives from the CNS parenchyma with signals coming from circulating immune cells, and to function as an on-alert gate for selective recruitment of inflammation-resolving leukocytes to the inflamed CNS parenchyma. Finally, we propose that functional dysregulation of the CP reflects a common underlying mechanism in the pathophysiology of neurodegenerative diseases, and can thus serve as a potential novel target for therapy.
Figures



References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–1705. - PMC - PubMed
-
- Bakalash S, Ben-Shlomo G, Aloni E, Shaked I, Wheeler L, Ofri R, Schwartz M. T-cell-based vaccination for morphological and functional neuroprotection in a rat model of chronically elevated intraocular pressure. J Mol Med. 2005;83:904–916. - PubMed
-
- Bakalash S, Kipnis J, Yoles E, Schwartz M. Resistance of retinal ganglion cells to an increase in intraocular pressure is immune-dependent. Invest Ophthalmol Vis Sci. 2002;43:2648–2653. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

