Post-marketing surveillance study with iodixanol in 20 185 Chinese patients from routine clinical practices
- PMID: 24357597
- PMCID: PMC4064546
- DOI: 10.1259/bjr.20130325
Post-marketing surveillance study with iodixanol in 20 185 Chinese patients from routine clinical practices
Abstract
Objective: To determine the incidence of immediate and delayed adverse drug reactions (ADRs), and to assess patient discomfort following administration of iodixanol during imaging examinations in routine clinical practice.
Methods: A total of 20 185 patients across 95 clinical centres were enrolled in a prospective post-marketing surveillance registry with iodixanol. Patients were monitored for occurrence of ADRs immediately following iodixanol administration and for up to 7 days after administration.
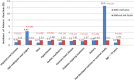
Results: The overall rate of ADRs was 1.52%, of which 0.58% was immediate and 0.97% was delayed onset. Two patients had non-fatal serious ADRs (0.01%). The ADRs were significantly more common in patients who underwent contrast-enhanced CT/coronary CT angiography vs others (p < 0.001), in those receiving pre-heated iodixanol vs non-heating (p < 0.001), in those aged 70 years or younger (p < 0.001), in those in whom a power injector was used for contrast delivery (p < 0.001) and in those with a history of an allergic reaction to contrast (p = 0.024). Multivariate analysis showed that female gender, intravenous route of contrast injection, body weight ≥ 80 kg, age less than 65 years, contrast flow rate ≥ 4 ml s⁻¹ and prior reaction to iodinated contrast medium were all significant and independent contributors to ADRs. Pre-treatment contrast volume and history of cardiac disease, gout, hypertension, diabetes mellitus or asthma did not affect the rate of ADRs. Discomfort was generally mild, with 94.8% of patients reporting a composite score of 0-3.
Conclusion: The safety of iodixanol in routine clinical practice was shown to be similar to the published safety profiles of other non-ionic iodinated contrast agents. Patient discomfort during administration was mild or absent in most patients.
Advances in knowledge: The major strength of this study is that it included 20 185 patients enrolled in various types of imaging examinations. The safety profile of iodixanol was comparable to previously published work.
Figures
Similar articles
-
Safety and patient comfort with iodixanol: a postmarketing surveillance study in 9515 patients undergoing diagnostic CT examinations.Acta Radiol. 2010 Oct;51(8):924-33. doi: 10.3109/02841851.2010.504739. Acta Radiol. 2010. PMID: 20828300
-
Safety of non-ionic contrast media in CT examinations for out-patients: retrospective multicenter analysis of 473,482 patients.Eur Radiol. 2024 Sep;34(9):5570-5577. doi: 10.1007/s00330-024-10654-2. Epub 2024 Mar 8. Eur Radiol. 2024. PMID: 38457038
-
Post-marketing surveillance of the safety profile of iodixanol in the outpatient CT setting: a prospective, multicenter, observational study of patient risk factors, adverse reactions and preventive measures in 9953 patients.Rofo. 2014 Nov;186(11):1028-34. doi: 10.1055/s-0034-1366370. Epub 2014 Apr 11. Rofo. 2014. PMID: 24729407
-
Safety, tolerance, and pharmacokinetics of iodixanol injection, a nonionic, isosmolar, hexa-iodinated contrast agent.Acad Radiol. 1996 Sep;3 Suppl 3:S475-84. doi: 10.1016/s1076-6332(05)80362-9. Acad Radiol. 1996. PMID: 8883524 Review.
-
Iodixanol versus iopromide in patients with renal insufficiency undergoing coronary angiography with or without PCI.Medicine (Baltimore). 2018 May;97(18):e0617. doi: 10.1097/MD.0000000000010617. Medicine (Baltimore). 2018. PMID: 29718868 Free PMC article. Review.
Cited by
-
Case Report: A Rare Case of Iodixanol-Induced Anaphylactic Shock in Cerebral Angiography.J Asthma Allergy. 2024 Apr 11;17:361-367. doi: 10.2147/JAA.S460263. eCollection 2024. J Asthma Allergy. 2024. PMID: 38623449 Free PMC article.
-
Cancer stratification by molecular imaging.Int J Mol Sci. 2015 Mar 4;16(3):4918-46. doi: 10.3390/ijms16034918. Int J Mol Sci. 2015. PMID: 25749472 Free PMC article. Review.
-
The incidence of skin lesions in contrast media-induced chemical hypersensitivity.Exp Ther Med. 2019 Feb;17(2):1113-1124. doi: 10.3892/etm.2018.7056. Epub 2018 Dec 5. Exp Ther Med. 2019. PMID: 30679982 Free PMC article.
-
Novel risk model for predicting acute adverse drug reactions following cardiac catheterization from TRUST study (The Safety and toleRability of UltraviSt in Patients Undergoing Cardiac CaTheterization).J Thorac Dis. 2019 Apr;11(4):1611-1620. doi: 10.21037/jtd.2019.04.66. J Thorac Dis. 2019. PMID: 31179105 Free PMC article.
-
The safety of iodixanol in interventional therapy for patients of different ages with cerebrovascular diseases.Am J Transl Res. 2021 Jul 15;13(7):8228-8234. eCollection 2021. Am J Transl Res. 2021. PMID: 34377310 Free PMC article.
References
-
- Michel MC. Post-marketing studies can make important contributions to medical knowledge. BMJ 2012; 345: e4740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical