Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon
- PMID: 24357601
- PMCID: PMC3912099
- DOI: 10.1104/pp.113.232678
Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon
Abstract
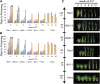
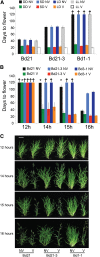
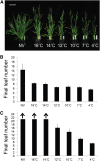
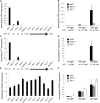
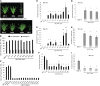
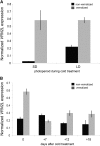
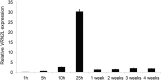
Timing of flowering is key to the reproductive success of many plants. In temperate climates, flowering is often coordinated with seasonal environmental cues such as temperature and photoperiod. Vernalization is an example of temperature influencing the timing of flowering and is defined as the process by which a prolonged exposure to the cold of winter results in competence to flower during the following spring. In cereals, three genes (VERNALIZATION1 [VRN1], VRN2, and FLOWERING LOCUS T [FT]) have been identified that influence the vernalization requirement and are thought to form a regulatory loop to control the timing of flowering. Here, we characterize natural variation in the vernalization and photoperiod responses in Brachypodium distachyon, a small temperate grass related to wheat (Triticum aestivum) and barley (Hordeum vulgare). Brachypodium spp. accessions display a wide range of flowering responses to different photoperiods and lengths of vernalization. In addition, we characterize the expression patterns of the closest homologs of VRN1, VRN2 (VRN2-like [BdVRN2L]), and FT before, during, and after cold exposure as well as in different photoperiods. FT messenger RNA levels generally correlate with flowering time among accessions grown in different photoperiods, and FT is more highly expressed in vernalized plants after cold. VRN1 is induced by cold in leaves and remains high following vernalization. Plants overexpressing VRN1 or FT flower rapidly in the absence of vernalization, and plants overexpressing VRN1 exhibit lower BdVRN2L levels. Interestingly, BdVRN2L is induced during cold, which is a difference in the behavior of BdVRN2L compared with wheat VRN2 during cold.
Figures

References
-
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 - PubMed
-
- Ahrens JL, Loomis WE. (1963) Floral induction and development in winter wheat. Crop Sci 3: 463–466
-
- Amasino R. (2010) Seasonal and developmental timing of flowering. Plant J 61: 1001–1013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

