Perfusion of ischemic brain in young and aged animals: a laser speckle flowmetry study
- PMID: 24357659
- PMCID: PMC3946827
- DOI: 10.1161/STROKEAHA.113.002944
Perfusion of ischemic brain in young and aged animals: a laser speckle flowmetry study
Abstract
Background and purpose: Aging is an important determinant of ischemic stroke outcomes. Both clinical and experimental stroke studies have shown that aging negatively correlates with infarct volumes but is associated with worsened functional recovery after stroke. This may correspond to a differing cellular and molecular response to stroke in the aged versus young brain. It was hypothesized in this study that the smaller injury seen in the aged ischemic brain is because of structural differences in microvasculature with aging or differences in intraischemic tissue perfusion.
Methods: Both young and aged C57BL6 mice were subject to middle cerebral artery occlusion modeling. Laser speckle flowmetry was used to study the functional dynamics of cerebral perfusion, and fluorescein isothiocyanate (FITC)-dextran staining was performed to examine the structural change in microvasculature. In separate cohorts, cresyl violet staining and immunohistochemistry with CD31 and IgG antibodies were applied to further assess the microvascular density and blood-brain barrier breakdown after stroke.
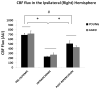
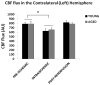
Results: No difference in cerebral blood flow was seen at the baseline, intraischemically, and postreperfusion in young versus aged mice. FITC-dextran and CD31 staining did not show significant differences in the microvascular density between young and aged ischemic brains. More extravasation of IgG through the blood-brain barrier was found in the young versus aged cohort at both 24 and 72 hours after stroke.
Conclusions: Cerebrovascular dynamics and perfusion are not responsible for the different stroke phenotypes seen in the young versus aged animals, which may be more related to different levels of blood-brain barrier breakdown.
Keywords: aging; brain ischemia; infarction; microcirculation; middle cerebral artery occlusion; perfusion; regional blood flow.
Conflict of interest statement
Figures












References
-
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146. - PubMed
-
- Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, et al. Functional recovery after ischemic stroke--a matter of age: Data from the austrian stroke unit registry. Neurology. 2012;78:279–285. - PubMed
-
- Rojas JI, Zurru MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke and transient ischemic attack in the very old--risk factor profile and stroke subtype between patients older than 80 years and patients aged less than 80 years. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2007;14:895–899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

