Estimating podocyte number and density using a single histologic section
- PMID: 24357669
- PMCID: PMC4005315
- DOI: 10.1681/ASN.2013080859
Estimating podocyte number and density using a single histologic section
Abstract
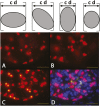
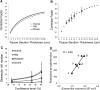
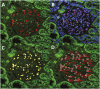
The reduction in podocyte density to levels below a threshold value drives glomerulosclerosis and progression to ESRD. However, technical demands prohibit high-throughput application of conventional morphometry for estimating podocyte density. We evaluated a method for estimating podocyte density using single paraffin-embedded formalin-fixed sections. Podocyte nuclei were imaged using indirect immunofluorescence detection of antibodies against Wilms' tumor-1 or transducin-like enhancer of split 4. To account for the large size of podocyte nuclei in relation to section thickness, we derived a correction factor given by the equation CF=1/(D/T+1), where T is the tissue section thickness and D is the mean caliper diameter of podocyte nuclei. Normal values for D were directly measured in thick tissue sections and in 3- to 5-μm sections using calibrated imaging software. D values were larger for human podocyte nuclei than for rat or mouse nuclei (P<0.01). In addition, D did not vary significantly between human kidney biopsies at the time of transplantation, 3-6 months after transplantation, or with podocyte depletion associated with transplant glomerulopathy. In rat models, D values also did not vary with podocyte depletion, but increased approximately 10% with old age and in postnephrectomy kidney hypertrophy. A spreadsheet with embedded formulas was created to facilitate individualized podocyte density estimation upon input of measured values. The correction factor method was validated by comparison with other methods, and provided data comparable with prior data for normal human kidney transplant donors. This method for estimating podocyte density is applicable to high-throughput laboratory and clinical use.
Copyright © 2014 by the American Society of Nephrology.
Figures


References
-
- Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 - PubMed
-
- Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 - PubMed
-
- Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 - PubMed
-
- Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

