Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers
- PMID: 24357672
- PMCID: PMC4033376
- DOI: 10.1681/ASN.2013080831
Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers
Abstract
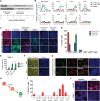
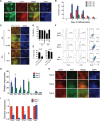
Human pluripotent stem cells (hPSCs) can generate a diversity of cell types, but few methods have been developed to derive cells of the kidney lineage. Here, we report a highly efficient system for differentiating human embryonic stem cells and induced pluripotent stem cells (referred to collectively as hPSCs) into cells expressing markers of the intermediate mesoderm (IM) that subsequently form tubule-like structures. Treatment of hPSCs with the glycogen synthase kinase-3β inhibitor CHIR99021 induced BRACHYURY(+)MIXL1(+) mesendoderm differentiation with nearly 100% efficiency. In the absence of additional exogenous factors, CHIR99021-induced mesendodermal cells preferentially differentiated into cells expressing markers of lateral plate mesoderm with minimal IM differentiation. However, the sequential treatment of hPSCs with CHIR99021 followed by fibroblast growth factor-2 and retinoic acid generated PAX2(+)LHX1(+) cells with 70%-80% efficiency after 3 days of differentiation. Upon growth factor withdrawal, these PAX2(+)LHX1(+) cells gave rise to apically ciliated tubular structures that coexpressed the proximal tubule markers Lotus tetragonolobus lectin, N-cadherin, and kidney-specific protein and partially integrated into embryonic kidney explant cultures. With the addition of FGF9 and activin, PAX2(+)LHX1(+) cells specifically differentiated into cells expressing SIX2, SALL1, and WT1, markers of cap mesenchyme nephron progenitor cells. Our findings demonstrate the effective role of fibroblast growth factor signaling in inducing IM differentiation in hPSCs and establish the most rapid and efficient system whereby hPSCs can be differentiated into cells with features characteristic of kidney lineage cells.
Copyright © 2014 by the American Society of Nephrology.
Figures



References
-
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 - PubMed
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM: Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147, 1998 - PubMed
-
- Ren X, Zhang J, Gong X, Niu X, Zhang X, Chen P, Zhang X: Differentiation of murine embryonic stem cells toward renal lineages by conditioned medium from ureteric bud cells in vitro. Acta Biochim Biophys Sin (Shanghai) 42: 464–471, 2010 - PubMed
-
- Kim D, Dressler GR: Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol 16: 3527–3534, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

