Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion
- PMID: 24357730
- PMCID: PMC3938152
- DOI: 10.1182/blood-2013-08-523001
Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion
Abstract
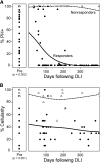
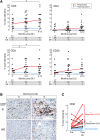
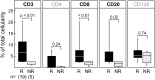
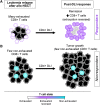
Increasing evidence across malignancies suggests that infiltrating T cells at the site of disease are crucial to tumor control. We hypothesized that marrow-infiltrating immune populations play a critical role in response to donor lymphocyte infusion (DLI), an established and potentially curative immune therapy whose precise mechanism remains unknown. We therefore analyzed marrow-infiltrating immune populations in 29 patients (22 responders, 7 nonresponders) with relapsed chronic myelogenous leukemia who received CD4(+) DLI in the pre-tyrosine kinase inhibitor era. Immunohistochemical analysis of pretreatment marrow revealed that the presence of >4% marrow-infiltrating CD8(+) (but not CD4(+)) T cells predicted DLI response, even in the setting of high leukemia burden. Furthermore, mRNA expression profiling of marrow-infiltrating T cells of a subset of responders compared with nonresponders revealed enrichment of T-cell exhaustion-specific genes in pretreatment T cells of DLI responders and significant downregulation of gene components in the same pathway in responders in conjunction with clinical response. Our data demonstrate that response to DLI is associated with quantity of preexisting marrow CD8(+) T cells and local reversal of T-cell exhaustion. Our studies implicate T-cell exhaustion as a therapeutic target of DLI and support the potential use of novel anti-PD1/PDL1 agents in lieu of DLI.
Figures


Comment in
-
Reversing CD8+ T-cell exhaustion with DLI.Blood. 2014 Feb 27;123(9):1289-90. doi: 10.1182/blood-2014-01-547000. Blood. 2014. PMID: 24578495 No abstract available.
References
-
- Kolb HJ, Mittermüller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–2465. - PubMed
-
- Kolb HJ, Schattenberg A, Goldman JM, et al. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. - PubMed
-
- Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–444. - PubMed
-
- Soiffer RJ. Donor lymphocyte infusions for acute myeloid leukaemia. Best Pract Res Clin Haematol. 2008;21(3):455–466. - PubMed
-
- Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11(4):473–487. - PubMed
Publication types
MeSH terms
Grants and funding
- 5R21CA115043-2/CA/NCI NIH HHS/United States
- T32 HG002295/HG/NHGRI NIH HHS/United States
- CA142106-06A1/CA/NCI NIH HHS/United States
- 5R01HL103532-03/HL/NHLBI NIH HHS/United States
- R01 CA183559/CA/NCI NIH HHS/United States
- P01 CA142106/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 HL103532/HL/NHLBI NIH HHS/United States
- P01 CA066996/CA/NCI NIH HHS/United States
- T32 CA009172/CA/NCI NIH HHS/United States
- R01 CA183560/CA/NCI NIH HHS/United States
- R01 CA155010/CA/NCI NIH HHS/United States
- R21 CA115043/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

