TRIM32-dependent transcription in adult neural progenitor cells regulates neuronal differentiation
- PMID: 24357807
- PMCID: PMC3877558
- DOI: 10.1038/cddis.2013.487
TRIM32-dependent transcription in adult neural progenitor cells regulates neuronal differentiation
Abstract
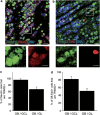
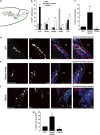
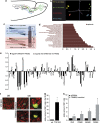
In the adult mammalian brain, neural stem cells in the subventricular zone continuously generate new neurons for the olfactory bulb. Cell fate commitment in these adult neural stem cells is regulated by cell fate-determining proteins. Here, we show that the cell fate-determinant TRIM32 is upregulated during differentiation of adult neural stem cells into olfactory bulb neurons. We further demonstrate that TRIM32 is necessary for the correct induction of neuronal differentiation in these cells. In the absence of TRIM32, neuroblasts differentiate slower and show gene expression profiles that are characteristic of immature cells. Interestingly, TRIM32 deficiency induces more neural progenitor cell proliferation and less cell death. Both effects accumulate in an overproduction of adult-generated olfactory bulb neurons of TRIM32 knockout mice. These results highlight the function of the cell fate-determinant TRIM32 for a balanced activity of the adult neurogenesis process.
Figures




Similar articles
-
The neural stem cell fate determinant TRIM32 regulates complex behavioral traits.Front Cell Neurosci. 2015 Mar 18;9:75. doi: 10.3389/fncel.2015.00075. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25852471 Free PMC article.
-
A complex of the ubiquitin ligase TRIM32 and the deubiquitinase USP7 balances the level of c-Myc ubiquitination and thereby determines neural stem cell fate specification.Cell Death Differ. 2019 Mar;26(4):728-740. doi: 10.1038/s41418-018-0144-1. Epub 2018 Jun 13. Cell Death Differ. 2019. PMID: 29899379 Free PMC article.
-
Expression of the Parkinson's Disease-Associated Gene Alpha-Synuclein is Regulated by the Neuronal Cell Fate Determinant TRIM32.Mol Neurobiol. 2017 Aug;54(6):4257-4270. doi: 10.1007/s12035-016-9989-9. Epub 2016 Jun 23. Mol Neurobiol. 2017. PMID: 27339877
-
Dynamic changes in the transcriptional profile of subventricular zone-derived postnatally born neuroblasts.Mech Dev. 2013 Jun-Aug;130(6-8):424-32. doi: 10.1016/j.mod.2012.11.003. Epub 2012 Dec 5. Mech Dev. 2013. PMID: 23220001 Review.
-
The heterogeneity of adult neural stem cells and the emerging complexity of their niche.Cold Spring Harb Symp Quant Biol. 2008;73:357-65. doi: 10.1101/sqb.2008.73.019. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022766 Review.
Cited by
-
The neural stem cell fate determinant TRIM32 regulates complex behavioral traits.Front Cell Neurosci. 2015 Mar 18;9:75. doi: 10.3389/fncel.2015.00075. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25852471 Free PMC article.
-
Tripartite Motif-Containing Protein 32 (TRIM32): What Does It Do for Skeletal Muscle?Cells. 2023 Aug 19;12(16):2104. doi: 10.3390/cells12162104. Cells. 2023. PMID: 37626915 Free PMC article. Review.
-
Parkinson's Disease-Associated Mutant LRRK2-Mediated Inhibition of miRNA Activity is Antagonized by TRIM32.Mol Neurobiol. 2018 Apr;55(4):3490-3498. doi: 10.1007/s12035-017-0570-y. Epub 2017 May 15. Mol Neurobiol. 2018. PMID: 28508149 Free PMC article.
-
p73 Regulates Primary Cortical Neuron Metabolism: a Global Metabolic Profile.Mol Neurobiol. 2018 Apr;55(4):3237-3250. doi: 10.1007/s12035-017-0517-3. Epub 2017 May 6. Mol Neurobiol. 2018. PMID: 28478509
-
TRIM24 protein promotes and TRIM32 protein inhibits cardiomyocyte hypertrophy via regulation of dysbindin protein levels.J Biol Chem. 2017 Jun 16;292(24):10180-10196. doi: 10.1074/jbc.M116.752543. Epub 2017 May 2. J Biol Chem. 2017. PMID: 28465353 Free PMC article.
References
-
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. - PubMed
-
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. - PubMed
-
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. - PubMed
-
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

