Suppression of soluble T cell-associated proteins by an anti-interferon-α monoclonal antibody in adult patients with dermatomyositis or polymyositis
- PMID: 24357810
- PMCID: PMC3970566
- DOI: 10.1093/rheumatology/ket413
Suppression of soluble T cell-associated proteins by an anti-interferon-α monoclonal antibody in adult patients with dermatomyositis or polymyositis
Abstract
Objective: The aim of this study was to identify serum markers that are modulated by an investigational anti-IFN-α mAb, sifalimumab, in adult DM or PM patients.
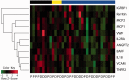
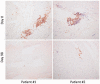
Methods: In a phase 1b clinical trial, sera were collected from a total of 48 DM or PM adult patients receiving either placebo for 3 months or sifalimumab for 6 months. Samples were tested for 128 selected proteins using a multiplex luminex immunoassay. Muscle biopsies from selected patients were stained for T cell infiltration using an anti-CD3 antibody.
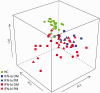
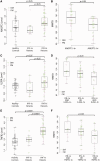
Results: A robust overexpression of multiple serum proteins in DM or PM patients was observed, particularly in patients with an elevated baseline type I IFN gene signature in the blood or muscle. Neutralization of the type I IFN gene signature by sifalimumab resulted in coordinated suppression of T cell-related proteins such as soluble IL-2RA, TNF receptor 2 (TNFR2) and IL-18. Muscle biopsies from two patients with the highest serum protein suppression were selected and found to have a pronounced reduction of muscle T cell infiltration. Down-regulation of IL-2RA correlated with favourable manual muscle test 8 (MMT-8) alterations in sifalimumab-dosed patients.
Conclusion: A reduced level of multiple T cell-associated proteins after sifalimumab but not placebo administration suggests a suppressive effect of blocking type I IFN signalling on T cell activation and chemoattraction that may lead to a reduction of T cell infiltration in the muscle of myositis patients. Further, soluble IL-2RA changes from baseline may serve as a responsive and/or predictive marker for type I IFN-targeted therapy in adult DM or PM patients.
Keywords: T cell infiltration; dermatomyositis; polymyositis; soluble interleukin-2 receptor; type I interferon.
Figures

References
-
- Dalakas MC. Review: an update on inflammatory and autoimmune myopathies. Neuropathol Appl Neurobiol. 2011;37:226–42. - PubMed
-
- Greenberg SA, Higgs BW, Morehouse C, et al. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun. 2011;13:207–13. - PubMed
-
- Bilgic H, Ytterberg SR, Amin S, et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009;60:3436–46. - PubMed
-
- Hengstman GJ, Vogels OJ, ter Laak HJ, et al. Myositis during long-term interferon-alpha treatment. Neurology. 2000;54:2186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

