IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation
- PMID: 24358127
- PMCID: PMC3864800
- DOI: 10.1371/journal.pone.0081774
IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation
Abstract
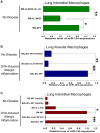
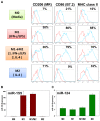
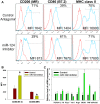
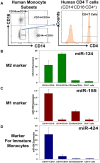
Monocytic cells exhibit a high level of heterogeneity and have two distinct modes of their activation: 1) classical M1 path associated with inflammation and tissue damage, and 2) alternative M2 path. Although it has been demonstrated that M2 macrophages play an important role in the regulation of the allergic immune responses, tissue maintenance and repair, little is known about the mechanisms that determine the M2 phenotype. We have previously shown that miR-124 is expressed in microglia that exhibit the M2 phenotype and overexpression of miR-124 in macrophages resulted in downregulation of a number of M1 markers (MHC class II, CD86) and up-regulation of several M2 markers (Fizz1, Arg1). We further investigated whether the polarization of macrophages towards the M2 phenotype induced miR-124 expression. We found that exposure of cells to IL-4 and IL-13 resulted in the upregulation of miR-124 in macrophages. We also demonstrated that IL-4 induced expression of three miR-124 precursor transcripts with predominant expression of pri-miR-124.3, suggesting regulation of miR-124 expression by IL-4 on a transcriptional level. Expression of miR-124 in microglia did not depend on IL-4 and/or IL-13, whereas expression of miR-124 in lung resident macrophages was IL-4 and IL-13-dependent and was upregulated by systemic administration of IL-4 or during allergic inflammation. Upregulation of several M2 markers (CD206, Ym1) and downregulation of the M1 markers (CD86, iNOS, TNF) in M2-polarized macrophages was abrogated by a miR-124 inhibitor, suggesting that this microRNA contributed to the M2 phenotype development and maintenance. Finally we showed that human CD14(+)CD16(+) intermediate monocytes, which are found in increased numbers in patients with allergies and bronchial asthma, expressed high levels of miR-124 and exhibited other properties of M2-like cells. Thus, our study suggests that miR-124 serves as a regulator of the M2 polarization in various subsets of monocytic cells both in vitro and in vivo.
Conflict of interest statement
Figures

References
-
- Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964. - PubMed
-
- Zhao C, Zhang H, Wong WC, Sem X, Han H, et al. (2009) Identification of novel functional differences in monocyte subsets using proteomic and transcriptomic methods. J Proteome Res 8: 4028–4038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

