PaCS is a novel cytoplasmic structure containing functional proteasome and inducible by cytokines/trophic factors
- PMID: 24358206
- PMCID: PMC3866174
- DOI: 10.1371/journal.pone.0082560
PaCS is a novel cytoplasmic structure containing functional proteasome and inducible by cytokines/trophic factors
Abstract
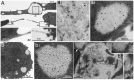
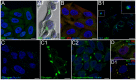
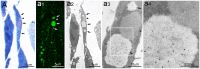
A variety of ubiquitinated protein-containing cytoplasmic structures has been reported, from aggresomes to aggresome-like induced structures/sequestosomes or particle-rich cytoplasmic structures (PaCSs) that we recently observed in some human diseases. Nevertheless, the morphological and cytochemical patterns of the different structures remain largely unknown thus jeopardizing their univocal identification. Here, we show that PaCSs resulted from proteasome and polyubiquitinated protein accumulation into well-demarcated, membrane-free, cytoskeleton-poor areas enriched in glycogen and glycosaminoglycans. A major requirement for PaCS detection by either electron or confocal microscopy was the addition of osmium to aldehyde fixatives. However, by analyzing living cells, we found that proteasome chymotrypsin-like activity concentrated in well-defined cytoplasmic structures identified as PaCSs by ultrastructural morphology and immunocytochemistry of the same cells. PaCSs differed ultrastructurally and cytochemically from sequestosomes which may coexist with PaCSs. In human dendritic or natural killer cells, PaCSs were induced in vitro by cytokines/trophic factors during differentiation/activation from blood progenitors. Our results provide evidence that PaCS is indeed a novel distinctive cytoplasmic structure which may play a critical role in the ubiquitin-proteasome system response to immune, infectious or proneoplastic stimuli.
Conflict of interest statement
Figures





Similar articles
-
Proteasome-Rich PaCS as an Oncofetal UPS Structure Handling Cytosolic Polyubiquitinated Proteins. In Vivo Occurrence, in Vitro Induction, and Biological Role.Int J Mol Sci. 2018 Sep 14;19(9):2767. doi: 10.3390/ijms19092767. Int J Mol Sci. 2018. PMID: 30223470 Free PMC article. Review.
-
Particulate cytoplasmic structures with high concentration of ubiquitin-proteasome accumulate in myeloid neoplasms.J Hematol Oncol. 2015 Jun 18;8:71. doi: 10.1186/s13045-015-0169-6. J Hematol Oncol. 2015. PMID: 26081257 Free PMC article.
-
Polyubiquitinated proteins, proteasome, and glycogen characterize the particle-rich cytoplasmic structure (PaCS) of neoplastic and fetal cells.Histochem Cell Biol. 2014 May;141(5):483-97. doi: 10.1007/s00418-014-1202-5. Epub 2014 Mar 1. Histochem Cell Biol. 2014. PMID: 24577783
-
Proteasome particle-rich structures are widely present in human epithelial neoplasms: correlative light, confocal and electron microscopy study.PLoS One. 2011;6(6):e21317. doi: 10.1371/journal.pone.0021317. Epub 2011 Jun 17. PLoS One. 2011. PMID: 21695063 Free PMC article.
-
Particle-rich cytoplasmic structure (PaCS): identification, natural history, role in cell biology and pathology.Biomolecules. 2014 Sep 22;4(3):848-61. doi: 10.3390/biom4030848. Biomolecules. 2014. PMID: 25247343 Free PMC article. Review.
Cited by
-
Phenotypic characteristics of peripheral immune cells of Myalgic encephalomyelitis/chronic fatigue syndrome via transmission electron microscopy: A pilot study.PLoS One. 2022 Aug 9;17(8):e0272703. doi: 10.1371/journal.pone.0272703. eCollection 2022. PLoS One. 2022. PMID: 35943990 Free PMC article.
-
Proteasome-Rich PaCS as an Oncofetal UPS Structure Handling Cytosolic Polyubiquitinated Proteins. In Vivo Occurrence, in Vitro Induction, and Biological Role.Int J Mol Sci. 2018 Sep 14;19(9):2767. doi: 10.3390/ijms19092767. Int J Mol Sci. 2018. PMID: 30223470 Free PMC article. Review.
-
Different Polyubiquitinated Bodies in Human Dendritic Cells: IL-4 Causes PaCS During Differentiation while LPS or IFNα Induces DALIS During Maturation.Sci Rep. 2017 May 12;7(1):1844. doi: 10.1038/s41598-017-02090-8. Sci Rep. 2017. PMID: 28500302 Free PMC article.
-
Particulate cytoplasmic structures with high concentration of ubiquitin-proteasome accumulate in myeloid neoplasms.J Hematol Oncol. 2015 Jun 18;8:71. doi: 10.1186/s13045-015-0169-6. J Hematol Oncol. 2015. PMID: 26081257 Free PMC article.
-
Polyubiquitinated proteins, proteasome, and glycogen characterize the particle-rich cytoplasmic structure (PaCS) of neoplastic and fetal cells.Histochem Cell Biol. 2014 May;141(5):483-97. doi: 10.1007/s00418-014-1202-5. Epub 2014 Mar 1. Histochem Cell Biol. 2014. PMID: 24577783
References
-
- Kuusisto E, Salminen A, Alafuzoff I (2001) Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport 12: 2085–2090. - PubMed
-
- Willis MS, Patterson C (2013) Proteotoxicity and cardiac dysfunction – Alzheimer's disease of the heart? N Engl J Med 368: 455–464. - PubMed
-
- Kopito RR (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10: 524–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

