Correlation of epididymal protease inhibitor and fibronectin in human semen
- PMID: 24358212
- PMCID: PMC3865146
- DOI: 10.1371/journal.pone.0082600
Correlation of epididymal protease inhibitor and fibronectin in human semen
Abstract
Objective: Epididymal protease inhibitor (Eppin) was located on the surface of spermatozoa and modulates the liquefaction of human semen. Here, we identify the correlative protein partner of Eppin to explore the molecular mechanism of liquefaction of human semen.
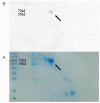
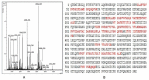
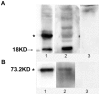
Methods: (1) Human seminal vesicle proteins were transferred on the membrane by Western blotting and separated by 2-D electrophoresis and incubated in recombinant Eppin. The correlative protein was identified by Mass Spectrometry (MS) (2). Western blotting was used to determine the relation of rEppin and rFibronectin(Fn); (3) Co-localization in spermatozoa were detected using immunofluorescence; (4) Correalation of Eppin and Fn was proved by co-immunoprecipitation.
Results: Fn was identified as the binding partner of recombinant Eppin by MS. Recombinant of Eppin was made and demonstrated that the Eppin fragment binds the fn 607-1265 fragment. The Eppin-Fn complex presents on the sperm tail and particularly in the midpiece region of human ejaculated spermatozoa. Immunoprecipitation indicated that Eppin in the spermatozoa lysates was complexed with Fn.
Conclusions: Our study demonstrates that Eppin and Fn bind to each other in human semen and on human ejaculated spermatozoa. Eppin-Fn complex may involve in semen coagulation, liquefaction and the survival and preparation of spermatozoa for fertility in the female reproductive tract.
Conflict of interest statement
Figures



Similar articles
-
[Correlation of epididymal protease inhibitor Eppin and Semenogelin on human ejaculated spermatozoa].Zhonghua Nan Ke Xue. 2006 May;12(5):428-31, 434. Zhonghua Nan Ke Xue. 2006. PMID: 16755874 Chinese.
-
Characterization of an eppin protein complex from human semen and spermatozoa.Biol Reprod. 2007 Sep;77(3):476-84. doi: 10.1095/biolreprod.107.060194. Epub 2007 Jun 13. Biol Reprod. 2007. PMID: 17567961
-
Association of eppin with semenogelin on human spermatozoa.Biol Reprod. 2005 May;72(5):1064-70. doi: 10.1095/biolreprod.104.036483. Epub 2004 Dec 8. Biol Reprod. 2005. PMID: 15590901
-
Functional studies of eppin.Biochem Soc Trans. 2011 Oct;39(5):1447-9. doi: 10.1042/BST0391447. Biochem Soc Trans. 2011. PMID: 21936831 Review.
-
Epididymal protein targets: a brief history of the development of epididymal protease inhibitor as a contraceptive.J Androl. 2011 Nov-Dec;32(6):698-704. doi: 10.2164/jandrol.110.012781. Epub 2011 Mar 25. J Androl. 2011. PMID: 21441428 Review.
Cited by
-
Proteomic signatures of infertile men with clinical varicocele and their validation studies reveal mitochondrial dysfunction leading to infertility.Asian J Androl. 2016 Mar-Apr;18(2):282-91. doi: 10.4103/1008-682X.170445. Asian J Androl. 2016. PMID: 26732106 Free PMC article.
-
Expression and correlation of male reproductive hormone levels with abnormal semen liquefaction time.Ann Med. 2025 Dec;57(1):2500692. doi: 10.1080/07853890.2025.2500692. Epub 2025 May 9. Ann Med. 2025. PMID: 40340744 Free PMC article.
-
Expressions of Semenogelin Gene in Male Syrian Hamsters according to Photoperiod.Dev Reprod. 2019 Dec;23(4):355-365. doi: 10.12717/DR.2019.23.4.355. Epub 2019 Dec 31. Dev Reprod. 2019. PMID: 31993541 Free PMC article.
-
The Expressional Pattern of Epididymal Protease Inhibitor (EPPIN) in the Male Syrian Hamsters.Dev Reprod. 2018 Sep;22(3):253-262. doi: 10.12717/DR.2018.22.3.253. Epub 2018 Sep 30. Dev Reprod. 2018. PMID: 30324162 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

