Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma
- PMID: 24358223
- PMCID: PMC3866194
- DOI: 10.1371/journal.pone.0082742
Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma
Abstract
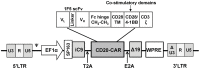
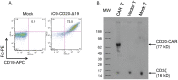
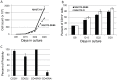
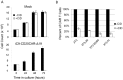
Modification of T cells with chimeric antigen receptors (CAR) has emerged as a promising treatment modality for human malignancies. Integration of co-stimulatory domains into CARs can augment the activation and function of genetically targeted T cells against tumors. However, the potential for insertional mutagenesis and toxicities due to the infused cells have made development of safe methods for removing transferred cells an important consideration. We have genetically modified human T cells with a lentiviral vector to express a CD20-CAR containing both CD28 and CD137 co-stimulatory domains, a "suicide gene" relying on inducible activation of caspase 9 (iC9), and a truncated CD19 selectable marker. Rapid expansion (2000 fold) of the transduced T cells was achieved in 28 days after stimulation with artificial antigen presenting cells. Transduced T cells exhibited effective CD20-specific cytotoxic activity in vitro and in a mouse xenograft tumor model. Activation of the iC9 suicide switch resulted in efficient removal of transduced T cells both in vitro and in vivo. Our work demonstrates the feasibility and promise of this approach for treating CD20(+) malignancies in a safe and more efficient manner. A phase I clinical trial using this approach in patients with relapsed indolent B-NHL is planned.
Conflict of interest statement
Figures



References
-
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, et al. (2012) B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood 119: 2709–2720 10.1182/blood-2011-10-384388 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

