Effects of reticuloendotheliosis virus infection on cytokine production in SPF chickens
- PMID: 24358317
- PMCID: PMC3865284
- DOI: 10.1371/journal.pone.0083918
Effects of reticuloendotheliosis virus infection on cytokine production in SPF chickens
Abstract
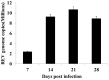
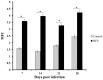
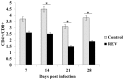
Infection with reticuloendotheliosis virus (REV), a gammaretrovirus in the Retroviridae family, can result in immunosuppression and subsequent increased susceptibility to secondary infections. The effects of REV infection on expression of mRNA for cytokine genes in chickens have not been completely elucidated. In this study, using multiplex branched DNA (bDNA) technology, we identified molecular mediators that participated in the regulation of the immune response during REV infection in chickens. Cytokine and chemokine mRNA expression levels were evaluated in the peripheral blood mononuclear cells (PBMCs). Expression levels of interleukin (IL)-4, IL-10, IL-13 and tumor necrosis factor (TNF)-α were significantly up-regulated while interferon (IFN)-α, IFN-β, IFN-γ, IL-1β, IL-2, IL-3, IL-15, IL-17F, IL-18 and colony-stimulating factor (CSF)-1 were markedly decreased in PBMCs at all stages of infection. Compared with controls, REV infected chickens showed greater expression levels of IL-8 in PBMCs 21 and 28 days post infection. In addition, REV regulates host immunity as a suppressor of T cell proliferative responses. The results in this study will help us to understand the host immune response to virus pathogens.
Conflict of interest statement
Figures

Similar articles
-
Changes in oxidation-antioxidation function on the thymus of chickens infected with reticuloendotheliosis virus.BMC Vet Res. 2020 Dec 11;16(1):483. doi: 10.1186/s12917-020-02708-6. BMC Vet Res. 2020. PMID: 33308224 Free PMC article.
-
Synergetic effects of subgroup J avian leukosis virus and reticuloendotheliosis virus co-infection on growth retardation and immunosuppression in SPF chickens.Vet Microbiol. 2014 Aug 27;172(3-4):425-31. doi: 10.1016/j.vetmic.2014.06.025. Epub 2014 Jul 3. Vet Microbiol. 2014. PMID: 25042879
-
Serological survey of Reticuloendotheliosis virus infection in chickens in China in 2005 to 2015.Poult Sci. 2017 Sep 1;96(11):3893-3895. doi: 10.3382/ps/pex209. Poult Sci. 2017. PMID: 29050414
-
Avian retroviruses.Vet Clin North Am Food Anim Pract. 1997 Mar;13(1):71-85. doi: 10.1016/s0749-0720(15)30365-0. Vet Clin North Am Food Anim Pract. 1997. PMID: 9071747 Review.
-
The avian reticuloendotheliosis virus, strain T (REV).Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1983 Sep;16(3):217-75. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1983. PMID: 6094120 Review. No abstract available.
Cited by
-
Analysis of the spleen proteome of chickens infected with reticuloendotheliosis virus.Arch Virol. 2017 May;162(5):1187-1199. doi: 10.1007/s00705-016-3180-5. Epub 2017 Jan 17. Arch Virol. 2017. PMID: 28097424 Free PMC article.
-
Isolation and full-genome sequence of two reticuloendotheliosis virus strains from mixed infections with Marek's disease virus in China.Virus Genes. 2015 Jun;50(3):418-24. doi: 10.1007/s11262-015-1191-z. Epub 2015 Apr 8. Virus Genes. 2015. PMID: 25850423
-
Comparative analysis of key immune protection factors in H9N2 avian influenza viruses infected and immunized specific pathogen-free chicken.Poult Sci. 2021 Jan;100(1):39-46. doi: 10.1016/j.psj.2020.09.080. Epub 2020 Oct 9. Poult Sci. 2021. PMID: 33357705 Free PMC article.
-
Allicin Alleviates Reticuloendotheliosis Virus-Induced Immunosuppression via ERK/Mitogen-Activated Protein Kinase Pathway in Specific Pathogen-Free Chickens.Front Immunol. 2017 Dec 22;8:1856. doi: 10.3389/fimmu.2017.01856. eCollection 2017. Front Immunol. 2017. PMID: 29312337 Free PMC article.
-
Transcriptional Profiling of Host Gene Expression in Chicken Embryo Fibroblasts Infected with Reticuloendotheliosis Virus Strain HA1101.PLoS One. 2015 May 14;10(5):e0126992. doi: 10.1371/journal.pone.0126992. eCollection 2015. PLoS One. 2015. PMID: 25973612 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

