Molecular and biochemical analyses of CbCel9A/Cel48A, a highly secreted multi-modular cellulase by Caldicellulosiruptor bescii during growth on crystalline cellulose
- PMID: 24358340
- PMCID: PMC3865294
- DOI: 10.1371/journal.pone.0084172
Molecular and biochemical analyses of CbCel9A/Cel48A, a highly secreted multi-modular cellulase by Caldicellulosiruptor bescii during growth on crystalline cellulose
Abstract
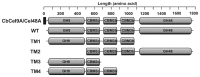
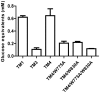
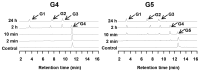
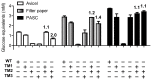
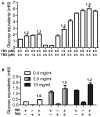
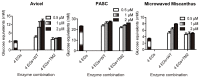
During growth on crystalline cellulose, the thermophilic bacterium Caldicellulosiruptor bescii secretes several cellulose-degrading enzymes. Among these enzymes is CelA (CbCel9A/Cel48A), which is reported as the most highly secreted cellulolytic enzyme in this bacterium. CbCel9A/Cel48A is a large multi-modular polypeptide, composed of an N-terminal catalytic glycoside hydrolase family 9 (GH9) module and a C-terminal GH48 catalytic module that are separated by a family 3c carbohydrate-binding module (CBM3c) and two identical CBM3bs. The wild-type CbCel9A/Cel48A and its truncational mutants were expressed in Bacillus megaterium and Escherichia coli, respectively. The wild-type polypeptide released twice the amount of glucose equivalents from Avicel than its truncational mutant that lacks the GH48 catalytic module. The truncational mutant harboring the GH9 module and the CBM3c was more thermostable than the wild-type protein, likely due to its compact structure. The main hydrolytic activity was present in the GH9 catalytic module, while the truncational mutant containing the GH48 module and the three CBMs was ineffective in degradation of either crystalline or amorphous cellulose. Interestingly, the GH9 and/or GH48 catalytic modules containing the CBM3bs form low-density particles during hydrolysis of crystalline cellulose. Moreover, TM3 (GH9/CBM3c) and TM2 (GH48 with three CBM3 modules) synergistically hydrolyze crystalline cellulose. Deletion of the CBM3bs or mutations that compromised their binding activity suggested that these CBMs are important during hydrolysis of crystalline cellulose. In agreement with this observation, seven of nine genes in a C. bescii gene cluster predicted to encode cellulose-degrading enzymes harbor CBM3bs. Based on our results, we hypothesize that C. bescii uses the GH48 module and the CBM3bs in CbCel9A/Cel48A to destabilize certain regions of crystalline cellulose for attack by the highly active GH9 module and other endoglucanases produced by this hyperthermophilic bacterium.
Conflict of interest statement
Figures

References
-
- Yang SJ, Kataeva I, Hamilton-Brehm SD, Engle NL, Tschaplinski TJ et al. (2009) Efficient degradation of lignocellulosic plant biomass, without pretreatment, by the thermophilic anaerobe "Anaerocellum thermophilum" DSM 6725. Appl Environ Microbiol 75: 4762-4769. doi: 10.1128/AEM.00236-09. PubMed: 19465524. - DOI - PMC - PubMed
-
- Blumer-Schuette SE, Lewis DL, Kelly RM (2010) Phylogenetic, microbiological, and glycoside hydrolase diversities within the extremely thermophilic, plant biomass-degrading genus Caldicellulosiruptor . Appl Environ Microbiol 76: 8084-8092. doi: 10.1128/AEM.01400-10. PubMed: 20971878. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

