Oxidative Lung Damage Resulting from Repeated Exposure to Radiation and Hyperoxia Associated with Space Exploration
- PMID: 24358450
- PMCID: PMC3866035
Oxidative Lung Damage Resulting from Repeated Exposure to Radiation and Hyperoxia Associated with Space Exploration
Abstract
Background: Spaceflight missions may require crewmembers to conduct Extravehicular Activities (EVA) for repair, maintenance or scientific purposes. Pre-breathe protocols in preparation for an EVA entail 100% hyperoxia exposure that may last for a few hours (5-8 hours), and may be repeated 2-3 times weekly. Each EVA is associated with additional challenges such as low levels of total body cosmic/galactic radiation exposure that may present a threat to crewmember health and therefore, pose a threat to the success of the mission. We have developed a murine model of combined, hyperoxia and radiation exposure (double-hit) in the context of evaluating countermeasures to oxidative lung damage associated with space flight. In the current study, our objective was to characterize the early and chronic effects of repeated single and double-hit challenge on lung tissue using a novel murine model of repeated exposure to low-level total body radiation and hyperoxia. This is the first study of its kind evaluating lung damage relevant to space exploration in a rodent model.
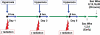
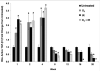
Methods: Mouse cohorts (n=5-15/group) were exposed to repeated: a) normoxia; b) >95% O2 (O2); c) 0.25Gy single fraction gamma radiation (IR); or d) a combination of O2 and IR (O2+IR) given 3 times per week for 4 weeks. Lungs were evaluated for oxidative damage, active TGFβ1 levels, cell apoptosis, inflammation, injury, and fibrosis at 1, 2, 4, 8, 12, 16, and 20 weeks post-initiation of exposure.
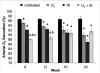
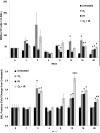
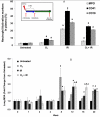
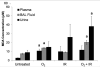
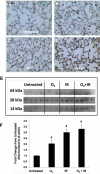
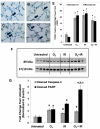
Results: Mouse cohorts exposed to all challenge conditions displayed decreased bodyweight compared to untreated controls at 4 and 8 weeks post-challenge initiation. Chronic oxidative lung damage to lipids (malondialdehyde levels), DNA (TUNEL, cleaved Caspase 3, cleaved PARP positivity) leading to apoptotic cell death and to proteins (nitrotyrosine levels) was elevated all treatment groups. Importantly, significant systemic oxidative stress was also noted at the late phase in mouse plasma, BAL fluid, and urine. Importantly, however, late oxidative damage across all parameters that we measured was significantly higher than controls in all cohorts but was exacerbated by the combined exposure to O2 and IR. Additionally, impaired levels of arterial blood oxygenation were noted in all exposure cohorts. Significant but transient elevation of lung tissue fibrosis (p<0.05), determined by lung hydroxyproline content, was detected as early as 2 week in mice exposed to challenge conditions and persisted for 4-8 weeks only. Interestingly, active TGFβ1 levels in +BAL fluid was also transiently elevated during the exposure time only (1-4 weeks). Inflammation and lung edema/lung injury was also significantly elevated in all groups at both early and late time points, especially the double-hit group.
Conclusion: We have characterized significant, early and chronic lung changes consistent with oxidative tissue damage in our murine model of repeated radiation and hyperoxia exposure relevant to space travel. Lung tissue changes, detectable several months after the original exposure, include significant oxidative lung damage (lipid peroxidation, DNA damage and protein nitrosative stress) and increased pulmonary fibrosis. These findings, along with increased oxidative stress in diverse body fluids and the observed decreases in blood oxygenation levels in all challenge conditions (whether single or in combination), lead us to conclude that in our model of repeated exposure to oxidative stressors, chronic tissue changes are detected that persist even months after the exposure to the stressor has ended. This data will provide useful information in the design of countermeasures to tissue oxidative damage associated with space exploration.
Keywords: Apoptosis; Bronchoalveolar lavage; Caspase 3; Double-hit; Extravehicular activity; Hyperoxia; Inflammation; Lung fibrosis; Lung injury; Mouse model; Nitrotyrosine; Oxidative stress; PARP; Radiation pneumonopathy; Space exploration; TGF-β1; TUNEL; Total body irradiation.
Figures
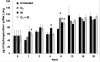

References
-
- Holloway RJ, Leong GF, Ainsworth EJ, Albright ML, Baum SJ. Recovery from radiation injury in the hamster as evaluated by the split-dose technique. USNRDL-TR-1111. Res Dev Tech Rep. 1967 - PubMed
-
- Hu S, Kim MH, McClellan GE, Cucinotta FA. Modeling the acute health effects of astronauts from exposure to large solar particle events. Health Phys. 2009;96:465–476. - PubMed
-
- Leong GF, Page NP, Ainsworth EJ, Hanks GE. Injury accumulation in sheep during protracted gamma radiation. USNRDL-TR-998. Res Dev Tech Rep . 1966 - PubMed
-
- Townsend LW. Implications of the space radiation environment for human exploration in deep space. Radiat Prot Dosimetry. 2005;115:44–50. - PubMed
-
- Prisk GK. The lung in space. Clin Chest Med. 2005;26:415–438. vi. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
