The oxidative burst reaction in mammalian cells depends on gravity
- PMID: 24359439
- PMCID: PMC3880029
- DOI: 10.1186/1478-811X-11-98
The oxidative burst reaction in mammalian cells depends on gravity
Abstract
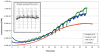
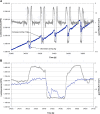
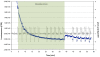
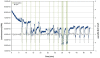
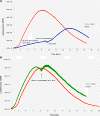
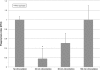
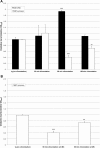
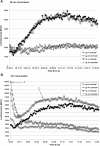
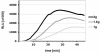
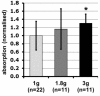
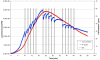
Gravity has been a constant force throughout the Earth's evolutionary history. Thus, one of the fundamental biological questions is if and how complex cellular and molecular functions of life on Earth require gravity. In this study, we investigated the influence of gravity on the oxidative burst reaction in macrophages, one of the key elements in innate immune response and cellular signaling. An important step is the production of superoxide by the NADPH oxidase, which is rapidly converted to H2O2 by spontaneous and enzymatic dismutation. The phagozytosis-mediated oxidative burst under altered gravity conditions was studied in NR8383 rat alveolar macrophages by means of a luminol assay. Ground-based experiments in "functional weightlessness" were performed using a 2 D clinostat combined with a photomultiplier (PMT clinostat). The same technical set-up was used during the 13th DLR and 51st ESA parabolic flight campaign. Furthermore, hypergravity conditions were provided by using the Multi-Sample Incubation Centrifuge (MuSIC) and the Short Arm Human Centrifuge (SAHC). The results demonstrate that release of reactive oxygen species (ROS) during the oxidative burst reaction depends greatly on gravity conditions. ROS release is 1.) reduced in microgravity, 2.) enhanced in hypergravity and 3.) responds rapidly and reversible to altered gravity within seconds. We substantiated the effect of altered gravity on oxidative burst reaction in two independent experimental systems, parabolic flights and 2D clinostat / centrifuge experiments. Furthermore, the results obtained in simulated microgravity (2D clinorotation experiments) were proven by experiments in real microgravity as in both cases a pronounced reduction in ROS was observed. Our experiments indicate that gravity-sensitive steps are located both in the initial activation pathways and in the final oxidative burst reaction itself, which could be explained by the role of cytoskeletal dynamics in the assembly and function of the NADPH oxidase complex.
Figures

Similar articles
-
Rapid adaptation to microgravity in mammalian macrophage cells.Sci Rep. 2017 Feb 27;7(1):43. doi: 10.1038/s41598-017-00119-6. Sci Rep. 2017. PMID: 28242876 Free PMC article.
-
Syk phosphorylation - a gravisensitive step in macrophage signalling.Cell Commun Signal. 2015 Feb 3;13:9. doi: 10.1186/s12964-015-0088-8. Cell Commun Signal. 2015. PMID: 25644261 Free PMC article.
-
Rapid Cellular Perception of Gravitational Forces in Human Jurkat T Cells and Transduction into Gene Expression Regulation.Int J Mol Sci. 2020 Jan 14;21(2):514. doi: 10.3390/ijms21020514. Int J Mol Sci. 2020. PMID: 31947583 Free PMC article.
-
Gravity as a biochemical determinant.Life Sci Space Res. 1979;17:147-60. doi: 10.1016/b978-0-08-023416-8.50024-6. Life Sci Space Res. 1979. PMID: 12008701 Review.
-
Perception and response to gravity in higher fungi--a critical appraisal.New Phytol. 1991;117:3-23. doi: 10.1111/j.1469-8137.1991.tb00940.x. New Phytol. 1991. PMID: 11541309 Review.
Cited by
-
MRTF may be the missing link in a multiscale mechanobiology approach toward macrophage dysfunction in space.Front Cell Dev Biol. 2022 Sep 12;10:997365. doi: 10.3389/fcell.2022.997365. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172272 Free PMC article.
-
Role of Apoptosis in Wound Healing and Apoptosis Alterations in Microgravity.Front Bioeng Biotechnol. 2021 Jun 17;9:679650. doi: 10.3389/fbioe.2021.679650. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34222218 Free PMC article. Review.
-
Effect of Oxidative Stress on Cardiovascular System in Response to Gravity.Int J Mol Sci. 2017 Jul 4;18(7):1426. doi: 10.3390/ijms18071426. Int J Mol Sci. 2017. PMID: 28677649 Free PMC article. Review.
-
Long-term space missions' effects on the human organism: what we do know and what requires further research.Front Physiol. 2024 Feb 13;15:1284644. doi: 10.3389/fphys.2024.1284644. eCollection 2024. Front Physiol. 2024. PMID: 38415007 Free PMC article. Review.
-
Rapid Morphological and Cytoskeletal Response to Microgravity in Human Primary Macrophages.Int J Mol Sci. 2019 May 15;20(10):2402. doi: 10.3390/ijms20102402. Int J Mol Sci. 2019. PMID: 31096581 Free PMC article.
References
-
- Cogoli A. Gravitational physiology of human immune cells: a review of in vivo, ex vivo and in vitro studies. J Gravit Physiol. 1996;11(1):1–9. - PubMed
-
- Bräucker R, Cogoli A, Hemmersbach R. In: Astrobiology: The Quest for the Conditions of Life. Baumstark-Khan C, Horneck GG, editor. Berlin: Heidelberg: Springer; 2001. Graviperception and graviresponse at the cellular level; pp. 284–297.
-
- Tauber S, Hauschild S, Crescio C, Secchi C, Paulsen K, Pantaleo A, Saba A, Buttron I, Thiel CS, Cogoli A, Pippia P, Ullrich O. Signal transduction in primary human T lymphocytes in altered gravity -results of the MASER-12 suborbital space flight mission. Cell Commun Signal. 2013;11(1):32. doi: 10.1186/1478-811X-11-32. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

