High-dose interferon-γ promotes abortion in mice by suppressing Treg and Th17 polarization
- PMID: 24359574
- PMCID: PMC4015477
- DOI: 10.1089/jir.2013.0062
High-dose interferon-γ promotes abortion in mice by suppressing Treg and Th17 polarization
Abstract
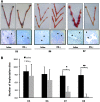
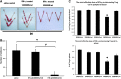
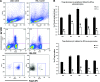
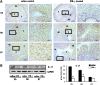
As a classic type I cytokine, interferon-gamma (IFN-γ) is known to manifest a miscarriage-inducing effect, although the specific mechanism is still unclear. To determine whether immune cells such as regulatory T (Treg) and Th17 cells are involved in these abortions, syngeneically pregnant (BALB/c×BALB/c) mice were subjected to intravaginal IFN-γ administration (5 × 10(3) IU/mouse on D3 of gestation). These mice experienced significant fetal loss on D7/D8 of pregnancy, and a remarkable drop in the Treg cell ratio was observed in the peripheral blood and the spleen by flow cytometry. In situ detection of the uterine tissue peri-implantation revealed that IFN-γ treatment also caused statistically significant reductions in forkhead box P3, RAR-related orphan receptor gamma, and IL-17 levels, which indicated local decreases in Treg and Th17 cells at uterine implantation sites. The IFN-γ receptor alpha (IFN-γRα) level was also lowered in the uterus. These results demonstrate that in murine pregnancy, a supraphysiological dose of IFN-γ could induce peri-implantation failure. Moreover, in this study, the decreases in both Treg and Th17-type cells, which may be relevant to the role of IFN-γRα, may be one of the main reasons that IFN-γ causes abortion.
Figures



References
-
- Aluvihare VR, Kallikourdis M, Betz AG. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5(3):266–271 - PubMed
-
- Arruvito L SM, Banham AH, Fainboim L. 2007. Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol 178(4):2572–2578 - PubMed
-
- Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, Wegmann TG. 1995. IL-10 prevents naturally occurring fetal loss in the CBA X DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol 154(9):4261–4268 - PubMed
-
- Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. 1990. Control of fetal survival in CBA X DBA/2 mice by lymphokine therapy. J Reprod Fertil 89(2):447–458 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

