Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function
- PMID: 24359596
- PMCID: PMC3896985
- DOI: 10.1186/1532-429X-15-107
Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function
Abstract
Background: Duchenne muscular dystrophy (DMD), an X-linked disorder affects approximately 1 in 5000 males, is universally associated with heart disease. We previously identified myocardial disease by late gadolinium enhancement (LGE) in DMD subjects at various stages of disease, but the true prevalence is unclear. Cardiovascular magnetic resonance (CMR) is well established for both assessment of ventricular function and myocardial fibrosis by LGE. We sought to establish i) prevalence and distribution of LGE in a large DMD population and ii) relationship among LGE, age, LVEF by CMR and current living status.
Methods: Current living status, demographic and CMR data including ventricular volumes, LVEF and LGE from 314 DMD patients undergoing evaluation at a single large tertiary referral center were analyzed.
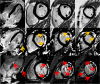
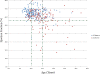
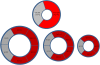
Results: 113 of 314 (36%) of DMD subjects showed LGE positivity with prevalence increasing from 17% of patients <10 years to 34% of those aged 10-15 years and 59% of those >15 years-old. Patients with LVEF ≥55% were LGE positive in 30% of cases; this increased to 84% for LVEF <55%. LGE was more prevalent in the free wall (531/1243, 42.7%) vs. septal segments (30/565, 5.3%). Patients with septal involvement were significantly older and had lower LVEF than those with isolated free wall LGE. Ten percent (11/113) patients who had LGE died 10.8 months after CMR. Only one patient from the LGE negative group died. Patients who died had higher heart rate, larger left ventricular volume and mass, greater number of positive LGE segment and increase incident of septal LGE compared to those who remained alive.
Conclusion: In DMD patients, LGE occurs early, is progressive and increases with both age and decreasing LVEF. Segmentally, the incidence of the number of positive LGE segments increase with age and lower LVEF. Older patients and those who died during the study period had more septal LGE involvement. The current studies suggest that the time course and distribution of LGE-positivity may be an important clinical biomarker to aid in the management of DMD-associated cardiac disease.
Figures

Similar articles
-
Variations in native T1 values in patients with Duchenne muscular dystrophy with and without late gadolinium enhancement.Int J Cardiovasc Imaging. 2021 Feb;37(2):635-642. doi: 10.1007/s10554-020-02031-z. Epub 2020 Sep 20. Int J Cardiovasc Imaging. 2021. PMID: 32951096
-
T1-Mapping and extracellular volume estimates in pediatric subjects with Duchenne muscular dystrophy and healthy controls at 3T.J Cardiovasc Magn Reson. 2020 Dec 10;22(1):85. doi: 10.1186/s12968-020-00687-z. J Cardiovasc Magn Reson. 2020. PMID: 33302967 Free PMC article.
-
Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in duchenne muscular dystrophy.J Am Heart Assoc. 2015 Mar 26;4(4):e001338. doi: 10.1161/JAHA.114.001338. J Am Heart Assoc. 2015. PMID: 25814625 Free PMC article.
-
Predictors of cardiac disease in duchenne muscular dystrophy: a systematic review and evidence grading.Orphanet J Rare Dis. 2024 Sep 28;19(1):359. doi: 10.1186/s13023-024-03372-x. Orphanet J Rare Dis. 2024. PMID: 39342355 Free PMC article.
-
Revisiting how we perform late gadolinium enhancement CMR: insights gleaned over 25 years of clinical practice.J Cardiovasc Magn Reson. 2023 Mar 16;25(1):18. doi: 10.1186/s12968-023-00925-0. J Cardiovasc Magn Reson. 2023. PMID: 36922844 Free PMC article. Review. No abstract available.
Cited by
-
Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy.Circulation. 2015 May 5;131(18):1590-8. doi: 10.1161/CIRCULATIONAHA.114.015151. Circulation. 2015. PMID: 25940966 Free PMC article. No abstract available.
-
Anti-Remodeling Cardiac Therapy in Patients With Duchenne Muscular Dystrophy, Meta-Analysis Study.Front Pharmacol. 2022 Jan 20;12:769896. doi: 10.3389/fphar.2021.769896. eCollection 2021. Front Pharmacol. 2022. PMID: 35126112 Free PMC article. Review.
-
Prognostic Utility of Cardiovascular Magnetic Resonance-Based Phenotyping in Patients With Muscular Dystrophy.J Am Heart Assoc. 2023 Nov 7;12(21):e030229. doi: 10.1161/JAHA.123.030229. Epub 2023 Nov 6. J Am Heart Assoc. 2023. PMID: 37929714 Free PMC article.
-
Cellular pathology of the human heart in Duchenne muscular dystrophy (DMD): lessons learned from in vitro modeling.Pflugers Arch. 2021 Jul;473(7):1099-1115. doi: 10.1007/s00424-021-02589-0. Epub 2021 Jun 24. Pflugers Arch. 2021. PMID: 34169350 Review.
-
Rate of Change in Cardiac Magnetic Resonance Imaging Measures Is Associated With Death in Duchenne Muscular Dystrophy.J Am Heart Assoc. 2024 May 7;13(9):e032960. doi: 10.1161/JAHA.123.032960. Epub 2024 Apr 30. J Am Heart Assoc. 2024. PMID: 38686878 Free PMC article.
References
-
- Online Mendelian Inheritance in Man, OMIM®. Baltimore, MD: Johns Hopkins University; MIM Number: {300377}: {11/28/12}: World Wide Web URL: http://www.omim.org/
-
- Fischmann A. et al.Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J. 2012;15(4):969–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

