Regulation of actin by ion-linked equilibria
- PMID: 24359734
- PMCID: PMC3882474
- DOI: 10.1016/j.bpj.2013.10.032
Regulation of actin by ion-linked equilibria
Abstract
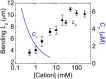
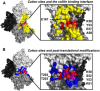
Actin assembly, filament mechanical properties, and interactions with regulatory proteins depend on the types and concentrations of salts in solution. Salts modulate actin through both nonspecific electrostatic effects and specific binding to discrete sites. Multiple cation-binding site classes spanning a broad range of affinities (nanomolar to millimolar) have been identified on actin monomers and filaments. This review focuses on discrete, low-affinity cation-binding interactions that drive polymerization, regulate filament-bending mechanics, and modulate interactions with regulatory proteins. Cation binding may be perturbed by actin post-translational modifications and linked equilibria. Partial cation occupancy under physiological and commonly used in vitro solution conditions likely contribute to filament mechanical heterogeneity and structural polymorphism. Site-specific cation-binding residues are conserved in Arp2 and Arp3, and may play a role in Arp2/3 complex activation and actin-filament branching activity. Actin-salt interactions demonstrate the relevance of ion-linked equilibria in the operation and regulation of complex biological systems.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Korn E.D., Carlier M.F., Pantaloni D. Actin polymerization and ATP hydrolysis. Science. 1987;238:638–644. - PubMed
-
- Pollard T.D., Borisy G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
-
- Pollard T.D., Blanchoin L., Mullins R.D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000;29:545–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

