Transmembrane protein (perfringolysin o) association with ordered membrane domains (rafts) depends upon the raft-associating properties of protein-bound sterol
- PMID: 24359745
- PMCID: PMC3882507
- DOI: 10.1016/j.bpj.2013.11.002
Transmembrane protein (perfringolysin o) association with ordered membrane domains (rafts) depends upon the raft-associating properties of protein-bound sterol
Abstract
Because transmembrane (TM) protein localization, or nonlocalization, in ordered membrane domains (rafts) is a key to understanding membrane domain function, it is important to define the origin of protein-raft interaction. One hypothesis is that a tight noncovalent attachment of TM proteins to lipids that have a strong affinity for ordered domains can be sufficient to induce raft-protein interaction. The sterol-binding protein perfringolysin O (PFO) was used to test this hypothesis. PFO binds both to sterols that tend to localize in ordered domains (e.g., cholesterol), and to those that do not (e.g., coprostanol), but it does not bind to epicholesterol, a raft-promoting 3α-OH sterol. Using a fluorescence resonance energy transfer assay in model membrane vesicles containing coexisting ordered and disordered lipid domains, both TM and non-TM forms of PFO were found to concentrate in ordered domains in vesicles containing high and low-Tm lipids plus cholesterol or 1:1 (mol/mol) cholesterol/epicholesterol, whereas they concentrate in disordered domains in vesicles containing high-Tm and low-Tm lipids plus 1:1 (mol/mol) coprostanol/epicholesterol. Combined with previous studies this behavior indicates that TM protein association with ordered domains is dependent upon both the association of the protein-bound sterol with ordered domains and hydrophobic match between TM segments and rafts.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

 ), epicholesterol (
), epicholesterol ( ), 1:1 cholesterol/epicholesterol (▾), or 1:1 coprostanol/epicholesterol (♢) were measured in PBS pH 5.1. Average (mean) values and SD values from triplicates are shown. Error bars are not shown where they are too small to illustrate.
), 1:1 cholesterol/epicholesterol (▾), or 1:1 coprostanol/epicholesterol (♢) were measured in PBS pH 5.1. Average (mean) values and SD values from triplicates are shown. Error bars are not shown where they are too small to illustrate.
 ), DMoPC with 45 mol % 1:1 coprostanol/epicholesterol (
), DMoPC with 45 mol % 1:1 coprostanol/epicholesterol ( ), 1:1 DSPC/DMoPC with 45 mol % cholesterol (▪), 1:1 DSPC/DMoPC with 45 mol % 1:1 cholesterol/epicholesterol (
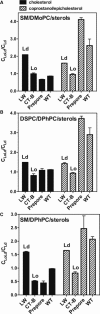
), 1:1 DSPC/DMoPC with 45 mol % cholesterol (▪), 1:1 DSPC/DMoPC with 45 mol % 1:1 cholesterol/epicholesterol ( ), or 1:1 DSPC/DMoPC with 45 mol % 1:1 coprostanol/epicholesterol (♦) were prepared in PBS pH 5.1. F samples contained both FRET donor (0.05 mol % pyrene-DPPE) and FRET acceptor (2 mol % Rho-DOPE). Fo samples only contained FRET donor (0.05 mol % pyrene-DPPE). The ratio of donor fluorescence in the presence of acceptor to that in its absence (F/Fo) is graphed. Average (mean) values and SD values from triplicates are shown. Abbreviations: chol = cholesterol; epichol = epicholesterol; and cop = coprostanol.
), or 1:1 DSPC/DMoPC with 45 mol % 1:1 coprostanol/epicholesterol (♦) were prepared in PBS pH 5.1. F samples contained both FRET donor (0.05 mol % pyrene-DPPE) and FRET acceptor (2 mol % Rho-DOPE). Fo samples only contained FRET donor (0.05 mol % pyrene-DPPE). The ratio of donor fluorescence in the presence of acceptor to that in its absence (F/Fo) is graphed. Average (mean) values and SD values from triplicates are shown. Abbreviations: chol = cholesterol; epichol = epicholesterol; and cop = coprostanol.

References
-
- Brown D.A., London E. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 1998;164:103–114. - PubMed
-
- Bijlmakers M.J. Protein acylation and localization in T cell signaling (Review) Mol. Membr. Biol. 2009;26:93–103. (Review) - PubMed
-
- Shogomori H., Hammond A.T., Brown D.A. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J. Biol. Chem. 2005;280:18931–18942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

