Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor
- PMID: 24360280
- PMCID: PMC4033691
- DOI: 10.1016/j.cell.2013.11.030
Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor
Abstract
Microglia are the resident macrophages of the CNS, and their functions have been extensively studied in various brain pathologies. The physiological roles of microglia in brain plasticity and function, however, remain unclear. To address this question, we generated CX3CR1(CreER) mice expressing tamoxifen-inducible Cre recombinase that allow for specific manipulation of gene function in microglia. Using CX3CR1(CreER) to drive diphtheria toxin receptor expression in microglia, we found that microglia could be specifically depleted from the brain upon diphtheria toxin administration. Mice depleted of microglia showed deficits in multiple learning tasks and a significant reduction in motor-learning-dependent synapse formation. Furthermore, Cre-dependent removal of brain-derived neurotrophic factor (BDNF) from microglia largely recapitulated the effects of microglia depletion. Microglial BDNF increases neuronal tropomyosin-related kinase receptor B phosphorylation, a key mediator of synaptic plasticity. Together, our findings reveal that microglia serve important physiological functions in learning and memory by promoting learning-related synapse formation through BDNF signaling.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
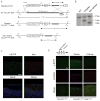



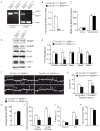

Comment in
-
Synaptic plasticity: a synaptic role for microglia.Nat Rev Neurosci. 2014 Feb;15(2):69. doi: 10.1038/nrn3678. Epub 2014 Jan 17. Nat Rev Neurosci. 2014. PMID: 24434913 No abstract available.
References
-
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. - PubMed
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. - PubMed
-
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nature Protocols. 2006;1:1306–1311. - PubMed
-
- Buch T, Heppner FL, Tertilt C, Heinen TJAJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Meth. 2005;2:419–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH067880/MH/NIMH NIH HHS/United States
- R01 NS047325/NS/NINDS NIH HHS/United States
- R01 NS030687/NS/NINDS NIH HHS/United States
- R01 NS087198/NS/NINDS NIH HHS/United States
- R01 MH096899/MH/NIMH NIH HHS/United States
- NS030687/NS/NINDS NIH HHS/United States
- F30 MH096370/MH/NIMH NIH HHS/United States
- P01 NS074972/NS/NINDS NIH HHS/United States
- P41 GM103533/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 MH96899/MH/NIMH NIH HHS/United States
- P01 AG031097/AG/NIA NIH HHS/United States
- R56 MH096899/MH/NIMH NIH HHS/United States
- F32 AG039127/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

